An AIF orthologue regulates apoptosis in yeast - PubMed (original) (raw)
. 2004 Sep 27;166(7):969-74.
doi: 10.1083/jcb.200404138. Epub 2004 Sep 20.
Paula Ludovico, Eva Herker, Sabrina Büttner, Silvia M Engelhardt, Thorsten Decker, Alexander Link, Astrid Proksch, Fernando Rodrigues, Manuela Corte-Real, Kai-Uwe Fröhlich, Joachim Manns, Céline Candé, Stephan J Sigrist, Guido Kroemer, Frank Madeo
Affiliations
- PMID: 15381687
- PMCID: PMC2172025
- DOI: 10.1083/jcb.200404138
An AIF orthologue regulates apoptosis in yeast
Silke Wissing et al. J Cell Biol. 2004.
Abstract
Apoptosis-inducing factor (AIF), a key regulator of cell death, is essential for normal mammalian development and participates in pathological apoptosis. The proapoptotic nature of AIF and its mode of action are controversial. Here, we show that the yeast AIF homologue Ynr074cp controls yeast apoptosis. Similar to mammalian AIF, Ynr074cp is located in mitochondria and translocates to the nucleus of yeast cells in response to apoptotic stimuli. Purified Ynr074cp degrades yeast nuclei and plasmid DNA. YNR074C disruption rescues yeast cells from oxygen stress and delays age-induced apoptosis. Conversely, overexpression of Ynr074cp strongly stimulates apoptotic cell death induced by hydrogen peroxide and this effect is attenuated by disruption of cyclophilin A or the yeast caspase YCA1. We conclude that Ynr074cp is a cell death effector in yeast and rename it AIF-1 (Aif1p, gene AIF1).
Figures
Figure 1.
Ynr074cp (Aif1p) is the yeast homologue of AIF. Alignment of Homo sapiens (Hs_AIF), Mus musculus (Mm_AIF), Dictyostelium discoideum (Dd_AIF), Caenorhabditis elegans (Ce_AIF) AIF, and Homo sapiens AMID (Hs_AMID) amino-acid sequences with the protein encoded by ORF YNR074C. Black boxes indicate amino acid identity and gray boxes indicate amino acid similarity.
Figure 2.
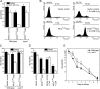
AIF translocates from mitochondria to the nucleus under apoptotic conditions, degrades DNA, and induces apoptosis in yeast. (A) Fluorescence microscopy of cells expressing Aif1pyEGFP during exponential growth. Mitochondria were visualized with mitochondrial marker DsRed Su1-69. (B) Fluorescence microscopy of exponentially growing cells expressing Aif1pyEGFP and DsRed-NLS (nuclear staining) after an apoptotic stimulus with 0.6 mM H2O2 for 5 h on SCD. (C) Fluorescence microscopy of chronological aged yeast cells expressing Aif1pyEGFP and DsRed-NLS (nuclear staining) after 5 d growing on SCD. (D) Immunoblot of cellular fractions of untreated cells during exponential growth expressing endogenous yEGFP-tagged AIF1 expressing (lanes 2, 4, 6) and controls (lanes 1, 3, 5). Blot probed with antibodies against GFP, or Cox2p, or with mAB 414, a mAb immunoreacting with both p110, a nuclear pore protein and with a 55-kD cytosolic protein (Aris and Blobel, 1989). (E) Import of in vitro–synthesized 35S-labeled Aif1p precursor into isolated mitochondria. Wild-type and Δtom5 mitochondria were incubated with Aif1p precursor protein. Wild-type mitochondria (M) and mitoplasts (MP) were treated with proteinase K (PK). (F) Survival of Δ_aif1_ and wild type after treatment with 0.4 mM H2O2 for 4 h during early exponential growth. Data represent mean ± SEM. (G) Survival of Aif1pFLAG overexpressor and vector control after a 20-h induction on galactose with and without H2O2. Data represent mean ± SEM. (H) TUNEL and DAPI staining of Aif1pFLAG overexpressor and vector control after a 20-h induction on galactose with H2O2. (I) Degradation of 1 μg purified plasmid DNA by 2.5 μg cell extracts from Aif1pFLAG overexpressor. (J) Purification of Aif1p after recombinant expression in E. coli under denaturing conditions, and after refolding via dialysis. IC, induced control; L, lysate; M, marker; FT, flow through; W, wash; E, eluate; and refolded Aif1p. (K) Time course of the degradation of isolated yeast nuclei by purified refolded Aif1p. (L) Degradation of 1 μg plasmid DNA with different concentrations of purified refolded Aif1p. Bars, 5 μm.
Figure 3.
Putative pathways of Aif1p death functions. (A) Survival of Aif1pFLAG overexpressor and vector control in wild type and yca1 disruptant background after a 20-h induction on galactose with and without 0.4 mM H2O2. Data represent mean ± SEM. (B) Aif1pFLAG overexpressor and vector control grown for 20 h on galactose with or without 0.4 mM H2O2 were analyzed in vivo for caspase activity by FITC-VAD-fmk staining using flow cytometry for quantification. (C) Survival of yeast cells upon moderate overexpression of Aif1pFLAG and vector control in wild type and Δ_cpr1_ background after a 20-h induction with or without 0.4 mM H2O2. Data represent mean ± SEM. (D) Survival of Aif1pFLAG overexpressor and vector control in wild type after a 20-h induction on galactose with or without 0.4 mM H2O2 and with 50 μg/ml Cyclosporin A (CsA) as indicated. Data represent mean ± SEM. (E) Chronological aging of wild type and Δ_aif1_ cells. Asterisks indicate a significant difference in an independent t test for days 3 and 5 at 0.02 and for day 7 at 0.075 level.
Similar articles
- Apoptosis-inducing factor is involved in the regulation of caspase-independent neuronal cell death.
Cregan SP, Fortin A, MacLaurin JG, Callaghan SM, Cecconi F, Yu SW, Dawson TM, Dawson VL, Park DS, Kroemer G, Slack RS. Cregan SP, et al. J Cell Biol. 2002 Aug 5;158(3):507-17. doi: 10.1083/jcb.200202130. Epub 2002 Jul 29. J Cell Biol. 2002. PMID: 12147675 Free PMC article. - p53-dependent caspase-2 activation in mitochondrial release of apoptosis-inducing factor and its role in renal tubular epithelial cell injury.
Seth R, Yang C, Kaushal V, Shah SV, Kaushal GP. Seth R, et al. J Biol Chem. 2005 Sep 2;280(35):31230-9. doi: 10.1074/jbc.M503305200. Epub 2005 Jun 27. J Biol Chem. 2005. PMID: 15983031 - Down-regulation of apoptosis-inducing factor protein by RNA interference inhibits UVA-induced cell death.
Yuan CQ, Li YN, Zhang XF. Yuan CQ, et al. Biochem Biophys Res Commun. 2004 May 14;317(4):1108-13. doi: 10.1016/j.bbrc.2004.03.169. Biochem Biophys Res Commun. 2004. PMID: 15094383 - Apoptosis-inducing factor (AIF): a ubiquitous mitochondrial oxidoreductase involved in apoptosis.
Daugas E, Nochy D, Ravagnan L, Loeffler M, Susin SA, Zamzami N, Kroemer G. Daugas E, et al. FEBS Lett. 2000 Jul 7;476(3):118-23. doi: 10.1016/s0014-5793(00)01731-2. FEBS Lett. 2000. PMID: 10913597 Review. - Apoptosis-inducing factor (AIF): a novel caspase-independent death effector released from mitochondria.
Candé C, Cohen I, Daugas E, Ravagnan L, Larochette N, Zamzami N, Kroemer G. Candé C, et al. Biochimie. 2002 Feb-Mar;84(2-3):215-22. doi: 10.1016/s0300-9084(02)01374-3. Biochimie. 2002. PMID: 12022952 Review.
Cited by
- Metabolic epistasis among apoptosis-inducing factor and the mitochondrial import factor CHCHD4.
Modjtahedi N, Hangen E, Gonin P, Kroemer G. Modjtahedi N, et al. Cell Cycle. 2015;14(17):2743-7. doi: 10.1080/15384101.2015.1068477. Epub 2015 Jul 15. Cell Cycle. 2015. PMID: 26178476 Free PMC article. - Targeting intrinsic cell death pathways to control fungal pathogens.
Kulkarni M, Stolp ZD, Hardwick JM. Kulkarni M, et al. Biochem Pharmacol. 2019 Apr;162:71-78. doi: 10.1016/j.bcp.2019.01.012. Epub 2019 Jan 17. Biochem Pharmacol. 2019. PMID: 30660496 Free PMC article. Review. - Calnexin regulates apoptosis induced by inositol starvation in fission yeast.
Guérin R, Beauregard PB, Leroux A, Rokeach LA. Guérin R, et al. PLoS One. 2009 Jul 16;4(7):e6244. doi: 10.1371/journal.pone.0006244. PLoS One. 2009. PMID: 19606215 Free PMC article. - Transcriptome analysis implicates secondary metabolite production, redox reactions, and programmed cell death during allorecognition in Cryphonectria parasitica.
Belov AA, Witte TE, Overy DP, Smith ML. Belov AA, et al. G3 (Bethesda). 2021 Jan 18;11(1):jkaa021. doi: 10.1093/g3journal/jkaa021. G3 (Bethesda). 2021. PMID: 33561228 Free PMC article. - The role of mitochondria in yeast programmed cell death.
Guaragnella N, Zdralević M, Antonacci L, Passarella S, Marra E, Giannattasio S. Guaragnella N, et al. Front Oncol. 2012 Jul 3;2:70. doi: 10.3389/fonc.2012.00070. eCollection 2012. Front Oncol. 2012. PMID: 22783546 Free PMC article.
References
- Candé, C., N. Vahsen, I. Kouranti, E. Schmitt, E. Daugas, C. Spahr, J. Luban, R.T. Kroemer, F. Giordanetto, C. Garrido, et al. 2004. AIF and cyclophilin A cooperate in apoptosis-associated chromatinolysis. Oncogene. 23:1514–1521. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases