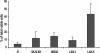A distinct "side population" of cells with high drug efflux capacity in human tumor cells - PubMed (original) (raw)
A distinct "side population" of cells with high drug efflux capacity in human tumor cells
C Hirschmann-Jax et al. Proc Natl Acad Sci U S A. 2004.
Abstract
A subset of stem cells, termed the "side population" (SP), has been identified in several tissues in mammalian species. These cells maintain a high efflux capability for antimitotic drugs. We have investigated whether functionally equivalent stem cells also may be detected in human cancers. We initially examined primary tumor cells from 23 patients with neuroblastoma and cell lines derived from a range of other tumors. A distinct SP was found in neuroblastoma cells from 15 of 23 patients (65%). The SP was capable of sustained expansion ex vivo and showed evidence for asymmetric division, generating both SP and non-SP progeny. These cells also expressed high levels of ABCG2 and ABCA3 transporter genes and had a greater capacity to expel cytotoxic drugs, such as mitoxantrone, resulting in better survival. A SP also was detected in breast cancer, lung cancer, and glioblastoma cell lines, suggesting that this phenotype defines a class of cancer stem cells with inherently high resistance to chemotherapeutic agents that should be targeted during the treatment of malignant disease.
Figures
Fig. 1.
Prevalence of Hoechstlow SP cells in neuroblastoma cell lines. Data shown are means ± SD of three to five independent experiments on each line.
Fig. 2.
Characteristic Hoechst 33342 dye staining profiles of two primary neuroblastoma specimens from patients with relapsed disease. (A) Female, 1.4 years old at diagnosis, MYCN-amplified, International Neuroblastoma Staging System stage IV. (B) Male, 7 years old at diagnosis, MYCN-amplified, stage IV. These two tumors illustrate the highest (A) and the lowest (B) proportions of SP cells observed. The SP pattern observed in the remaining patients with intermediate value was identical.
Fig. 3.
Increased expression of c-kit stem cell factor receptor (A) and GD2 (B) on the SP cells of patients with relapsed neuroblastoma. P ≤ 0.05 for GD2 and c-kit stem cell factor receptor. Solid line, SP; dashed line, non-SP; dotted line, control.
Fig. 4.
SP cells from neuroblastoma cell lines become SP and non-SP cells, whereas non-SP cells remain non-SP (illustrated for SK-N-SH). Hoechst 33342 dye-stained neuroblastoma cells (A) were sorted for the SP and non-SP fraction, and each subpopulation was cultivated. After 6 weeks, the SP (B) and non-SP (C) were stained with Hoechst dye and reanalyzed.
Fig. 5.
Relative expression of ATP-binding cassette transporter genes in neuroblastoma SP and non-SP cells by quantitative real-time RT-PCR amplification analysis. For quantification of gene expression, amplification of 18S ribosomal RNA has been performed as an endogenous control to standardize the amount of sample. The amount of target, normalized to the endogenous reference and relative to a calibrator, is given by 2–ΔΔCT. [The threshold cycle (CT) indicates the fractional cycle number at which the amount of amplified target reaches a fixed threshold.] Shown are the representative results of three different experiments. SD < 5%. For ABCG2 and ABCA3, P = 0.01, whereas the P value for MDR1 was not significant.
Fig. 6.
Efflux and survival of mitoxantrone from neuroblastoma SP cells. (A) Efflux of mitoxantrone in primary neuroblastoma samples from five patients (nos. 1–5) with relapsed disease after incubation with Hoechst 33342 dye and mitoxantrone. Fluorescence was plotted for three gated cell populations: SP (solid line), non-SP (dashed line), and control (dotted line) (P < 0.05). (B) JF and SK-N-SH lines were cultured in the presence of 0, 1, or 10 ng/ml mitoxantrone for 3 days, then analyzed for the SP cells. (C) Fifty SP or non-SP cells were sorted in six replicates into 96-well plates and cultured with 1 ng/ml mitoxantrone. After 14 days of culture, the number of colonies formed in each well was counted (*, P < 0.05).
Fig. 7.
Characteristic Hoechst 33342 dye SPs discerned in four tumor cell lines. Plots for Ewing sarcoma RD-ES (A), teratocarcinoma PA-1 (B), breast adenocarcinoma SK-BR-3 (C), and small-cell lung cancer NCI-H146 (D) are shown.
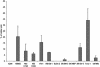
Fig. 8.
Overall prevalence of Hoechstlow SP cells in 12 tumor cell lines. Data shown are means ± SD of three to five independent experiments. See Table 3 for the key to the origin of the cell lines.
Similar articles
- The multidrug resistance transporter ABCG2 (breast cancer resistance protein 1) effluxes Hoechst 33342 and is overexpressed in hematopoietic stem cells.
Kim M, Turnquist H, Jackson J, Sgagias M, Yan Y, Gong M, Dean M, Sharp JG, Cowan K. Kim M, et al. Clin Cancer Res. 2002 Jan;8(1):22-8. Clin Cancer Res. 2002. PMID: 11801536 - Analysis of ABCG2 expression and side population identifies intrinsic drug efflux in the HCC cell line MHCC-97L and its modulation by Akt signaling.
Hu C, Li H, Li J, Zhu Z, Yin S, Hao X, Yao M, Zheng S, Gu J. Hu C, et al. Carcinogenesis. 2008 Dec;29(12):2289-97. doi: 10.1093/carcin/bgn223. Epub 2008 Sep 26. Carcinogenesis. 2008. PMID: 18820285 - [Analysis of the characteristics of side population cells in the human ovarian cancer cell line OVCAR-3].
Luo LJ, Zhao Z, Zeng JF, Liang B, Yang JX, Cao DY, Shen K. Luo LJ, et al. Zhonghua Fu Chan Ke Za Zhi. 2012 Apr;47(4):281-5. Zhonghua Fu Chan Ke Za Zhi. 2012. PMID: 22781115 Chinese. - Detection and characterization of side population in Ewing's sarcoma SK-ES-1 cells in vitro.
Yang M, Zhang R, Yan M, Ye Z, Liang W, Luo Z. Yang M, et al. Biochem Biophys Res Commun. 2010 Jan 1;391(1):1062-6. doi: 10.1016/j.bbrc.2009.12.020. Epub 2009 Dec 10. Biochem Biophys Res Commun. 2010. PMID: 20004177 - ABCG2: the key to chemoresistance in cancer stem cells?
An Y, Ongkeko WM. An Y, et al. Expert Opin Drug Metab Toxicol. 2009 Dec;5(12):1529-42. doi: 10.1517/17425250903228834. Expert Opin Drug Metab Toxicol. 2009. PMID: 19708828 Review.
Cited by
- Benefits of Zebrafish Xenograft Models in Cancer Research.
Chen X, Li Y, Yao T, Jia R. Chen X, et al. Front Cell Dev Biol. 2021 Feb 11;9:616551. doi: 10.3389/fcell.2021.616551. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33644052 Free PMC article. Review. - Cancer stem cell hypothesis and gastric carcinogenesis: Experimental evidence and unsolved questions.
Rocco A, Compare D, Nardone G. Rocco A, et al. World J Gastrointest Oncol. 2012 Mar 15;4(3):54-9. doi: 10.4251/wjgo.v4.i3.54. World J Gastrointest Oncol. 2012. PMID: 22468184 Free PMC article. - Transitional dynamics of cancer stem cells in invasion and metastasis.
Richard V, Kumar TRS, Pillai RM. Richard V, et al. Transl Oncol. 2021 Jan;14(1):100909. doi: 10.1016/j.tranon.2020.100909. Epub 2020 Oct 10. Transl Oncol. 2021. PMID: 33049522 Free PMC article. Review. - Side population cells from human melanoma tumors reveal diverse mechanisms for chemoresistance.
Luo Y, Ellis LZ, Dallaglio K, Takeda M, Robinson WA, Robinson SE, Liu W, Lewis KD, McCarter MD, Gonzalez R, Norris DA, Roop DR, Spritz RA, Ahn NG, Fujita M. Luo Y, et al. J Invest Dermatol. 2012 Oct;132(10):2440-2450. doi: 10.1038/jid.2012.161. Epub 2012 May 24. J Invest Dermatol. 2012. PMID: 22622430 Free PMC article. - Identification of MicroRNAs With In Vivo Efficacy in Multiple Myeloma-related Xenograft Models.
Weidle UH, Nopora A. Weidle UH, et al. Cancer Genomics Proteomics. 2020 Jul-Aug;17(4):321-334. doi: 10.21873/cgp.20192. Cancer Genomics Proteomics. 2020. PMID: 32576578 Free PMC article. Review.
References
- Goodell, M. A., Rosenzweig, M., Kim, H., Marks, D. F., DeMaria, M., Paradis, G., Grupp, S. A., Sieff, C. A., Mulligan, R. C. & Johnson, R. P. (1997) Nat. Med. 3, 1337–1345. - PubMed
- Gussoni, E., Soneoka, Y., Strickland, C. D., Buzney, E. A., Khan, M. K., Flint, A. F., Kunkel, L. M. & Mulligan, R. C. (1999) Nature 401, 390–394. - PubMed
- Zhou, S., Schuetz, J. D., Bunting, K. D., Colapietro, A.-M., Sampath, J., Morris, J. J., Lagutina, I., Grosveld, G. C., Osawa, M., Nakauchi, H. & Sorrentino, B. P. (2001) Nat. Med. 7, 1028–1034. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 CA094237/CA/NCI NIH HHS/United States
- T32 HL092332/HL/NHLBI NIH HHS/United States
- T32 HL092332-06/HL/NHLBI NIH HHS/United States
- R01 CA78792/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources