MAPK-dependent regulation of IL-1- and beta-adrenoreceptor-induced inflammatory cytokine production from mast cells: implications for the stress response - PubMed (original) (raw)
MAPK-dependent regulation of IL-1- and beta-adrenoreceptor-induced inflammatory cytokine production from mast cells: implications for the stress response
David S Chi et al. BMC Immunol. 2004.
Abstract
Background: Catecholamines, such as epinephrine, are elaborated in stress responses, and mediate vasoconstriction to cause elevation in systemic vascular resistance and blood pressure. Our previous study has shown that IL-1 can induce mast cells to produce proinflammatory cytokines which are involved in atherogenesis. The aim of this study was to determine the effects of epinephrine on IL-1-induced proatherogenic cytokine production from mast cells.
Results: Two ml of HMC-1 (0.75 x 106 cells/ml) were cultured with epinephrine (1 x 10-5 M) in the presence or absence of IL-1 beta (10 ng/ml) for 24 hrs. HMC-1 cultured alone produced none to trace amounts of IL-6, IL-8, and IL-13. IL-1 beta significantly induced production of these cytokines in HMC-1, while epinephrine alone did not. However, IL-6, IL-8, and IL-13 production induced by IL-1 beta were significantly enhanced by addition of epinephrine. The enhancing effect appears to involve NF-kappa B and p38 MAPK pathways. Flow cytometry showed the presence of beta1 and beta2 adrenoreceptors on resting mast cells. The enhancing effect of proatherogenic cytokine production by epinephrine was down regulated by the beta1 and beta2 adrenoceptor antagonist, propranolol, but not by the beta1 adrenoceptor antagonist, atenolol, suggesting the effect involved beta2 adrenoceptors. The enhancing effect of epinephrine on proatherogenic cytokine production was also down regulated by the immunosuppressive drug, dexamethasone.
Conclusions: These results not only confirm that an acute phase cytokine, IL-1 beta, regulates mast cell function, but also show that epinephrine up regulates the IL-1 beta induction of proatherogenic cytokines in mast cells. These data provide a novel role for epinephrine, a stress hormone, in inflammation and atherogenesis.
Figures
Figure 1
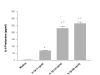
IL-1β induces IL-6 production from HMC-1 cells. To each well of a 6 well culture plate, two ml of HMC-1 mast cells (0.75 × 106cells/ml) were cultured with IL-1β (1, 10, and 50 ng/ml) for 24 hours. The cultures were carried out in triplicate. Supernatants were harvested for measuring IL-6 by ELISA. By Student's t-test analysis, * indicates p < 0.0001, when compared with the medium alone. + indicates p < 0.0005, when compared with the IL-1 (1 ng/ml) group.
Figure 2
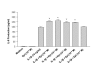
Enhancing effect of epinephrine on IL-6 production from IL-1β-induced HMC-1 cells. To each well of a 6 well culture plate, two ml of HMC-1 mast cells (0.75 × 106 cells/ml) were cultured with epinephrine (1 × 10-3 to 1 × 10-7 M) in the presence and absence of IL-1β (10 ng/ml) for 24 hrs in triplicate. Supernatants were harvested for measuring IL-6 by ELISA. By Student's t-test analysis, * indicates p < 0.05, when compared with the IL-1β-treated group.
Figure 3
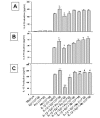
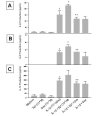
Effect of propranolol (Pro) and atenolol (Ate) on the enhancing effect of epinephrine (Epi) on production of IL-6 (A), IL-8 (B), and IL-13 (C) from IL-1β-induced HMC-1 cells. To each well of a 6 well culture plate, two ml of HMC-1 mast cells (0.75 × 106 cells/ml) were cultured alone (Medium), or in the presence of IL-1β (10 ng/ml), Epi (1 × 10-5 M), Pro (1 × 10-4 to 1 × 10-6 M), Ate (1 × 10-4 to 1 × 10-6 M), and the combinations of these reagents for 24 hrs in triplicate. Supernatants were harvested for measuring IL-6, IL-8, and IL-13 by ELISA. IL-8 and IL-13 production were not detected in the Medium, Epi, Pro, and Ate alone groups. In A and B, by Student's t-test analysis, * and + indicate p < 0.05, when compared with the IL-1β-treated group, and the IL-1β plus Epi group, respectively. In C, * indicates p < 0.01, when compared with the IL-1β-treated group; p values for ++, +, ##, and # were <0.00005, <0.0005, <0.01, and <0.05, when compared with the IL-1β plus Epi group.
Figure 4
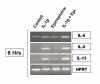
RT-PCR analysis for IL-6, IL-8, and IL-13 in HMC-1 treated with IL-1β and epinephrine. HMC-1 were treated for 6 hours with IL-1β with and without epinephrine and harvested for RNA preparation. RNA was subjected to RT-PCR with specific primers for target genes. HPRT was used as a house keeping gene to ensure equal loading. IL-6 gene expression was increased with IL-1β treatment and further increased with IL-1β plus epinephrine. Epinephrine alone had no effect on IL-6 gene expression in HMC-1. IL-8 and IL-13 showed similar results with a more robust expression of gene transcripts at this time point.
Figure 5
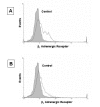
Detection of β1 and β2 adrenergic receptors on HMC-1 cell by flow cytometry analysis. Resting HMC-1 were harvested and stained with a purified rabbit polyclonal antibody to either β1 or β2 adrenergic receptor and counter stained with a secondary goat anti-rabbit FITC conjugated antibody. Normal rabbit serum and the FITC conjugated goat anti-rabbit Ig G antibody was used as a staining control.
Figure 6
Effect of dexamethasone (Dex) on the enhancing effect of epinephrine (Epi) on production of IL-6 (A), IL-8 (B), and IL-13 (C) from IL-1β-induced HMC-1 cells. To each well of a 6 well culture plate, two ml of HMC-1 mast cells (0.75 × 106 cells/ml) were cultured alone (Medium), or in the presence of IL-1β (10 ng/ml), Epi (1 × 10-5 M), Dex (1 × 10-7 M), and the combinations of these reagents for 24 hrs in triplicate. Supernatants were harvested for measuring IL-6, IL-8, and IL-13 by ELISA. * p < 0.005, when compared with the medium control, + p < 0.05 compared to the IL-1β-treated group, and ++ p < 0.05 compared to the IL-1β plus Epi group.
Figure 7
Effects of IL-1β and epinephrine on NF-κB translocation in HMC-1. HMC-1 were treated for 1 and 2 hours with IL-1β and epinephrine. NF-κB translocation was analyzed by a shift in oligonucleotide binding in EMSA gels. After one hour of treatment, NF-κB translocation is increased in the IL-1β treated cells but not in the untreated or epinephrine treated cells. Addition of IL-1β plus epinephrine does not further enhance NF-κB translocation. After two hours of treatment, NF-κB translocation in HMC-1 starts to decrease.
Figure 8
Phosphorylated and total p38 MAPK in HMC-1 cells treated with IL-1β, epinephrine, and IL-1β plus epinephrine. HMC-1 were treated for 30 minutes with the indicated reagents and harvested for phosphorylated p38 expression by Western blot. Unphosphorylated p38 was used as loading control to show total MAPK expression. IL-1β treated cells showed a small amount of p38 activation while the bulk of p38 was activated with epinephrine. IL-1β plus epinephrine had no additional effects over epinephrine alone.
Figure 9
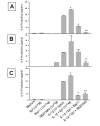
Effect of Bay 11 and SB203580 on the enhancing effect of epinephrine (Epi) on production of IL-6 (A), IL-8 (B), and IL-13 (C) from IL-1β-induced HMC-1 cells. To each well of a 6 well culture plate, two ml of HMC-1 mast cells (0.75 × 106 cells/ml) were cultured alone (Medium), or in the presence of IL-1β (10 ng/ml), Epi (1 × 10-5 M), Bay 11 (1 × 10-5 M), SB 203580 (1 × 10-5 M), and the combinations of these reagents for 24 hrs in triplicate. Supernatants were harvested for measuring IL-6, IL-8, and IL-13 by ELISA. IL-8 production was not detected in the Medium, Epi, Bay 11 alone groups, while IL-13 production was not detected in the Bay 11 and SB 203580 alone groups. In A, by Student's t-test analysis, * indicates p < 0.005, when compared with the IL-1β-treated group, and + and ++ indicate p < 0.0005 and <0.00005, when compared with the IL-1β plus Epi group. In B, * indicates p < 0.05, when compared with both the IL-1β-treated group, and the IL-1β plus Epi group. In C, * indicates p < 0.005, when compared with the IL-1β-treated group, and + and ++ indicate p < 0.00005 and <0.0001, when compared with the IL-1β plus Epi group.
Figure 10
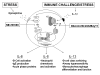
Schematic presentation showing the possible route of IL-6, IL-8, and IL-13 signaling. Endogenous IL-1β production may occur with immune challenge by cytokines, bacteria, and viruses, and any microtrauma in the body while epinephrine is released in states of stress or sympathetic nervous system activation. The pathways activated by these signals converge on IL-6, IL-8, and IL-13 genes to induce cytokine production that is greater than either signal alone. IL-1β activates the NF-κB pathway which leads to significant amounts of IL-6, IL-8, and IL-13 production. Epinephrine activates the p38 MAPK pathway which may activate other transcription factors or stabilize the IL-6, 8, and 13 mRNA. From our data it is evident that IL-1β and epinephrine do not combine to further activate NF-κB or the promotor activity of the IL-13 gene. The importance of IL-6, IL-8, and IL-13 are listed in the figure.
Similar articles
- Th2 cytokine production from mast cells is directly induced by lipopolysaccharide and distinctly regulated by c-Jun N-terminal kinase and p38 pathways.
Masuda A, Yoshikai Y, Aiba K, Matsuguchi T. Masuda A, et al. J Immunol. 2002 Oct 1;169(7):3801-10. doi: 10.4049/jimmunol.169.7.3801. J Immunol. 2002. PMID: 12244175 - Involvement of mitogen-activated protein kinase and NF-kappaB activation in Ca2+-induced IL-8 production in human mast cells.
Kim MS, Lim WK, Park RK, Shin T, Yoo YH, Hong SH, An NH, Kim HM. Kim MS, et al. Cytokine. 2005 Dec 7;32(5):226-33. doi: 10.1016/j.cyto.2005.10.001. Epub 2005 Dec 15. Cytokine. 2005. PMID: 16343928 - Human lung fibroblasts express interleukin-6 in response to signaling after mast cell contact.
Fitzgerald SM, Lee SA, Hall HK, Chi DS, Krishnaswamy G. Fitzgerald SM, et al. Am J Respir Cell Mol Biol. 2004 Apr;30(4):585-93. doi: 10.1165/rcmb.2003-0282OC. Epub 2003 Oct 17. Am J Respir Cell Mol Biol. 2004. PMID: 14565941 - Activated Mast Cells Mediate Low-Grade Inflammation in Type 2 Diabetes: Interleukin-37 Could Be Beneficial.
Conti P, Ronconi G, Kritas SK, Caraffa A, Theoharides TC. Conti P, et al. Can J Diabetes. 2018 Oct;42(5):568-573. doi: 10.1016/j.jcjd.2018.01.008. Epub 2018 Jan 31. Can J Diabetes. 2018. PMID: 29885882 Review. - Mast Cells May Regulate The Anti-Inflammatory Activity of IL-37.
Theoharides TC, Tsilioni I, Conti P. Theoharides TC, et al. Int J Mol Sci. 2019 Jul 29;20(15):3701. doi: 10.3390/ijms20153701. Int J Mol Sci. 2019. PMID: 31362339 Free PMC article. Review.
Cited by
- Unpredictable stress delays recovery from exercise-induced muscle pain: contribution of the sympathoadrenal axis.
Alvarez P, Green PG, Levine JD. Alvarez P, et al. Pain Rep. 2019 Sep 18;4(5):e782. doi: 10.1097/PR9.0000000000000782. eCollection 2019 Sep-Oct. Pain Rep. 2019. PMID: 31875187 Free PMC article. - β2-and β3-Adrenergic Receptors Contribute to Cancer-Evoked Pain in a Mouse Model of Osteosarcoma via Modulation of Neural Macrophages.
Bruno G, De Logu F, Souza Monteiro de Araujo D, Subbiani A, Lunardi F, Rettori S, Nassini R, Favre C, Calvani M. Bruno G, et al. Front Pharmacol. 2021 Sep 27;12:697912. doi: 10.3389/fphar.2021.697912. eCollection 2021. Front Pharmacol. 2021. PMID: 34646131 Free PMC article. - Catechol-O-methyltransferase inhibition increases pain sensitivity through activation of both beta2- and beta3-adrenergic receptors.
Nackley AG, Tan KS, Fecho K, Flood P, Diatchenko L, Maixner W. Nackley AG, et al. Pain. 2007 Apr;128(3):199-208. doi: 10.1016/j.pain.2006.09.022. Epub 2006 Nov 7. Pain. 2007. PMID: 17084978 Free PMC article. - Sustained stimulation of β2- and β3-adrenergic receptors leads to persistent functional pain and neuroinflammation.
Zhang X, Hartung JE, Bortsov AV, Kim S, O'Buckley SC, Kozlowski J, Nackley AG. Zhang X, et al. Brain Behav Immun. 2018 Oct;73:520-532. doi: 10.1016/j.bbi.2018.06.017. Epub 2018 Jun 20. Brain Behav Immun. 2018. PMID: 29935309 Free PMC article. - Paternal restraint stress affects offspring metabolism via ATF-2 dependent mechanisms in Drosophila melanogaster germ cells.
Seong KH, Ly NH, Katou Y, Yokota N, Nakato R, Murakami S, Hirayama A, Fukuda S, Kang S, Soga T, Shirahige K, Ishii S. Seong KH, et al. Commun Biol. 2020 May 4;3(1):208. doi: 10.1038/s42003-020-0935-z. Commun Biol. 2020. PMID: 32367035 Free PMC article.
References
- Krishnaswamy G, Kelley J, Johnson D, Youngberg G, Stone W, Huang SK, Bieber J, Chi DS. The human mast cell: functions in physiology and disease. Front Biosci. 2001;6:D1109–D1127. - PubMed
- Cannon JG, Evans WJ, Hughes VA, Meredith CN, Dinarello CA. Physiological mechanisms contributing to increased interleukin-1 secretion. J Appl Physiol. 1986;61:1869–1874. - PubMed
- Biasucci LM, Vitelli A, Liuzzo G, Altamura S, Caligiuri G, Monaco C, Rebuzzi AG, Ciliberto G, Maseri A. Elevated levels of interleukin-6 in unstable angina. Circulation. 1996;94:874–877. - PubMed
- Paul H, Whitaker JH, Downs CJ, Alturjuman AM, Martin E, Krishnaswamy G, Khan AA, Hiremagular S, Cantor K, Chi DS. Plasma interleukin-6, fibrinogen levels, and fibrinogen promoter polymorphism in patients with coronary artery disease. Chest. 1997;112:S67S.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources