The glycan-rich outer layer of the cell wall of Mycobacterium tuberculosis acts as an antiphagocytic capsule limiting the association of the bacterium with macrophages - PubMed (original) (raw)
The glycan-rich outer layer of the cell wall of Mycobacterium tuberculosis acts as an antiphagocytic capsule limiting the association of the bacterium with macrophages
Richard W Stokes et al. Infect Immun. 2004 Oct.
Abstract
Mycobacterium tuberculosis, the causative agent of tuberculosis, is a facultative intracellular pathogen that infects macrophages and other host cells. We show that sonication of M. tuberculosis results in the removal of material from the surface capsule-like layer of the bacteria, resulting in an enhanced propensity of the bacteria to bind to macrophages. This effect is observed with disparate murine and human macrophage populations though, interestingly, not with freshly explanted alveolar macrophages. Enhanced binding to macrophages following sonication is significantly greater within members of the M. tuberculosis family (pathogens) than within the Mycobacterium avium complex (opportunistic pathogens) or for Mycobacterium smegmatis (saprophyte). Sonication does not affect the viability or the surface hydrophobicity of M. tuberculosis but does result in changes in surface charge and in the binding of mannose-specific lectins to the bacterial surface. The increased binding of sonicated M. tuberculosis was not mediated through complement receptor 3. These results provide evidence that the surface capsule on members of the M. tuberculosis family may be an important virulence factor involved in the survival of M. tuberculosis in the mammalian host. They also question the view that M. tuberculosis is readily ingested by any macrophage it encounters and support the contention that M. tuberculosis, like many other microbial pathogens, has an antiphagocytic capsule that limits and controls the interaction of the bacterium with macrophages.
Figures
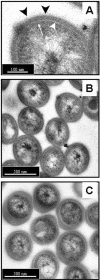
FIG. 1.
Transmission electron microscopy of M. tuberculosis stained with ruthenium red to demonstrate the capsule. The bacteria had been syringed (A and B) or sonicated in three 30-s bursts (C) prior to processing. (A) The cell wall envelope is shown and consists of the plasma membrane and peptidoglycan layers (thin white arrow), an electron-transparent region (large white arrowhead) representing the mycolic acids, glycolipids, and other lipid polymers, and a thick capsule-like outer layer stained strongly by the ruthenium red (blackarrowheads). (B) Syringed bacteria exhibit a capsule of uniform thickness and distribution among the population. (C) Following sonication, the capsule appears to have extended and is unevenly distributed, both around each individual bacteria and between individuals in the population. However, the integrity of the bacterial cells otherwise appears to be comparable to that of the syringed bacteria in B. Bars: 100 nm (A) and 500 nm (B and C).
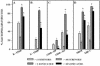
FIG. 2.
Association of syringed and sonicated (three 30-s bursts) M. tuberculosis, strain Erdman with various macrophage populations was assessed and is expressed as a percentage of the macrophage population that was infected with one or more syringed bacteria (>1 syringed), 10 or more syringed bacteria (>10 syringed), one or more sonicated bacteria (>1 sonicated), and 10 or more sonicated bacteria (>10 sonicated). The mean ± standard error of the mean are shown for (A) murine resident peritoneal macrophages (R-PMΦ), 13 experiments representing a total of 35 replicate coverslips; (B) thioglycolate-elicited peritoneal macrophages (E- PMΦ), four experiments representing a total of eight replicate coverslips; (C) freshly explanted alveolar macrophages at day zero (AMΦ-D0) or in vitro-differentiated alveolar macrophages at day 4 (AMΦ-D4), four experiments representing a total of 12 replicate coverslips; and (D) human monocyte-derived macrophages (MDM) or macrophage-like cells (THP-1), three experiments representing a total of nine replicate coverslips. The MOI was 20 bacteria per macrophage for all macrophage types (A, B, and D) except alveolar macrophages (C), for whichthe MOI was 500:1. *, P < 0.0001, and #, P < 0.05, when comparing sonicated to syringed bacteria.
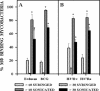
FIG. 3.
Association of syringed and sonicated (three 30-s bursts) members of the M. tuberculosis family with mouse resident peritoneal macrophages was assessed and is expressed as the percentage of macrophages binding more than 10 or more than 30 syringed or sonicated bacteria. Erdman and H37Rv are virulent strains of M. tuberculosis. H37Ra is an avirulent strain of M. tuberculosis. BCG is an avirulent vaccine strain of M. bovis and is closely related to M. tuberculosis. The mean ± standard error of the mean are shown for (A) two experiments representing a total of six replicate coverslips at an MOI of 10:1 and (B) three experiments representing a total of nine replicate coverslips at an MOI of 20:1. *, P < 0.0001, and $, P < 0.01, when comparing sonicated to syringed bacteria in the same group.
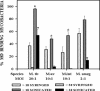
FIG. 4.
Association of different species of syringed and sonicated (three 30-s bursts) mycobacteria with mouse resident peritoneal macrophages was assessed and is expressed as the percentage of macrophages binding more than 10 or more than 30 syringed or sonicated bacteria. M. tuberculosis strain Erdman (M. tb), mouse-virulent M. avium (M.av), mouse-virulent M. intracellulare (M. int), and M. smegmatis (M.smeg), a saprophytic mycobacterium, were tested at the MOIs shown. The mean ± standard error of the mean are shown for three experiments representing a total of seven coverslips. ○, P < 0.001, and #, P < 0.05, when comparing sonicated to syringed bacteria in the same group.
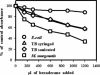
FIG. 5.
Hydrophobicity of syringed and sonicated (three 30-s bursts) M. tuberculosis strain Erdman compared to that of M. smegmatis and E. coli. Bacteria were suspended in an aqueous buffer and mixed with a range of hexadecane concentrations as indicated. Following separation of the two phases, the optical density at 400 nm (OD400) of the aqueous phase was measured. Results from one experiment are expressed as a percentage of the control absorbance, which was the optical density at 400 nm of bacteria in the aqueous buffer alone.
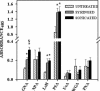
FIG. 6.
Lectin binding of untreated, syringed, and sonicated (three 30-s bursts) M. tuberculosis strain Erdman was assessed in a lectin enzyme-linked immunosorbent assay. Binding of biotinylated lectins to immobilized M. tuberculosis strain Erdman was quantitated. Each bar is the mean ± standard error of the mean from three separate experiments, each with triplicate readings. Lectin nomenclature and specificity are described in Materials and Methods. *, P < 0.05 when comparing syringed or sonicated bacteria with untreated bacteria. $, P < 0.05 when comparing sonicated with syringed bacteria.
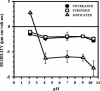
FIG. 7.
Microelectrophoresis measurements of the mobility of untreated, syringed, and sonicated (three 30-s bursts) M. bovis, BCG was measured in 150 mM NaCl over a range of pHs. Mobility was calculated from the average velocity, the applied voltage, and the electrical length. Each point is the average of 10 readings ± standard error of the mean.
Similar articles
- Pulmonary surfactant protein A mediates enhanced phagocytosis of Mycobacterium tuberculosis by a direct interaction with human macrophages.
Gaynor CD, McCormack FX, Voelker DR, McGowan SE, Schlesinger LS. Gaynor CD, et al. J Immunol. 1995 Dec 1;155(11):5343-51. J Immunol. 1995. PMID: 7594549 - The capsule of Mycobacterium tuberculosis and its implications for pathogenicity.
Daffé M, Etienne G. Daffé M, et al. Tuber Lung Dis. 1999;79(3):153-69. doi: 10.1054/tuld.1998.0200. Tuber Lung Dis. 1999. PMID: 10656114 Review. - Survival mechanisms of pathogenic Mycobacterium tuberculosis H37Rv.
Meena LS, Rajni. Meena LS, et al. FEBS J. 2010 Jun;277(11):2416-27. doi: 10.1111/j.1742-4658.2010.07666.x. FEBS J. 2010. PMID: 20553485 Review.
Cited by
- Mycobacteria emulsified in olive oil-in-water trigger a robust immune response in bladder cancer treatment.
Noguera-Ortega E, Blanco-Cabra N, Rabanal RM, Sánchez-Chardi A, Roldán M, Guallar-Garrido S, Torrents E, Luquin M, Julián E. Noguera-Ortega E, et al. Sci Rep. 2016 Jun 6;6:27232. doi: 10.1038/srep27232. Sci Rep. 2016. PMID: 27265565 Free PMC article. - Offense and Defense in Granulomatous Inflammation Disease.
Wang X, Liu Y. Wang X, et al. Front Cell Infect Microbiol. 2022 Jun 29;12:797749. doi: 10.3389/fcimb.2022.797749. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35846773 Free PMC article. Review. - The role of hydrophobicity in tuberculosis evolution and pathogenicity.
Jankute M, Nataraj V, Lee OY, Wu HHT, Ridell M, Garton NJ, Barer MR, Minnikin DE, Bhatt A, Besra GS. Jankute M, et al. Sci Rep. 2017 May 2;7(1):1315. doi: 10.1038/s41598-017-01501-0. Sci Rep. 2017. PMID: 28465507 Free PMC article. - Mycobacterium marinum MgtC plays a role in phagocytosis but is dispensable for intracellular multiplication.
Belon C, Gannoun-Zaki L, Lutfalla G, Kremer L, Blanc-Potard AB. Belon C, et al. PLoS One. 2014 Dec 29;9(12):e116052. doi: 10.1371/journal.pone.0116052. eCollection 2014. PLoS One. 2014. PMID: 25545682 Free PMC article. - The role of scavenger receptor B1 in infection with Mycobacterium tuberculosis in a murine model.
Schäfer G, Guler R, Murray G, Brombacher F, Brown GD. Schäfer G, et al. PLoS One. 2009 Dec 24;4(12):e8448. doi: 10.1371/journal.pone.0008448. PLoS One. 2009. PMID: 20041149 Free PMC article.
References
- Berger, M., T. M. Norvell, M. F. Tosi, S. E. Emancipator, M. W. Konstan, and J. R. Schreiber. 1993. Tissue-specific Fcg and complement receptor expression by alveolar macrophages determines relative importance of IgG and complement in promoting phagocytosis of Pseudomonas aeruginosa. Paediatr. Res. 35:68-77. - PubMed
- Bermudez, L. E., F. J. Sangari, P. Kolonoski, M. Petrofsky, and J. Goodman. 2002. The efficiency of the translocation of Mycobacterium tuberculosis across a bilayer of epithelial and endothelial cells as a model of the alveolar wall is a consequence of transport within mononuclear phagocytes and invasion of alveolar epithelial cells. Infect. Immun. 70:140-146. - PMC - PubMed
- Besra, G. S. 1998. Preparation of cell wall fractions from mycobacteria, p. 91-107. In T. Parish and N. G. Stoker (ed.) Methods in molecular biology: mycobacteria protocols. Humana Press, Totowa, N.J. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources