Chronic haloperidol promotes corticostriatal long-term potentiation by targeting dopamine D2L receptors - PubMed (original) (raw)
Chronic haloperidol promotes corticostriatal long-term potentiation by targeting dopamine D2L receptors
Diego Centonze et al. J Neurosci. 2004.
Erratum in
- J Neurosci. 2005 Jan 26;25(4):1034-5
Abstract
Reduced glutamate-mediated synaptic transmission has been implicated in the pathophysiology of schizophrenia. Because antipsychotic agents might exert their beneficial effects against schizophrenic symptoms by strengthening excitatory transmission in critical dopaminoceptive brain areas, in the present study we have studied the effects of acute and chronic haloperidol treatment on striatal synaptic plasticity. Repetitive stimulation of corticostriatal terminals in slices induced either long-term depression or long-term potentiation (LTP) of excitatory transmission in control rats, whereas it invariably induced NMDA receptor-dependent LTP in animals treated chronically with haloperidol. Haloperidol effects were mimicked and occluded in mice lacking both D2L and D2S isoforms of dopamine D2 receptors (D2R-/-), in mice lacking D2L receptors and expressing normal levels of D2S receptors (D2R-/-;D2L-/-), and in mice lacking D2L receptors and overexpressing D2S receptors (D2L-/-). These data indicate that the blockade of D2L receptors was responsible for the LTP-favoring action of haloperidol in the striatum. In contrast, overexpression of D2S receptors uncovered a facilitatory role of this receptor isoform in LTP formation because LTP recorded from D2L-/- mice, but not those recorded from wild-type, D2R-/-, and D2R-/-;D2L-/- mice, was insensitive to the pharmacological blockade of D1 receptors. The identification of the cellular, molecular, and receptor mechanisms involved in the action of haloperidol in the brain is essential to understand how antipsychotic agents exert their beneficial and side effects.
Figures
Figure 1.
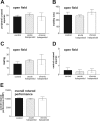
Lack of gross motor abnormalities in rats treated with haloperidol. A-D, Both acute (1 d) and chronic (20 d) haloperidol treatment (1 mg/kg) failed to alter horizontal (A, B, D) and vertical (C) motor activity measured in an open field. E, Overall rotarod performance was also unaffected by the two regimens of treatment.
Figure 2.
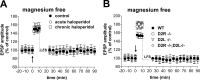
Chronic but not acute haloperidol treatment alters corticostriatal synaptic plasticity. A, The graph shows that in the presence of 1.2 m
m
external magnesium (LTD protocol), HFS (arrow) of corticostriatal fibers induced LTD of glutamatergic transmission in striatal neurons recorded from sham-treated (control) and 1-d-treated (acute haloperidol) rats. Conversely, the same stimulation protocol induced LTP in rats treated chronically (20 d) with haloperidol. B, HFS delivered in the absence of magnesium ions from the bathing solution (LTP protocol) induced LTP in sham-treated, acute, and chronic haloperidol-treated rats. Traces in the bottom part of the figure are intracellular recordings from single experiments showing EPSPs 10 min before (pre) and 30 min after (post) HFS in control and chronic haloperidol-treated rats, in the presence (A) or in the absence (B) of external magnesium. In this figure and in the following ones, the resting membrane potential and input resistance of the recorded neurons were constant and ranged between -83 ± 5 mV and 48 ± 16 MΩ.
Figure 3.
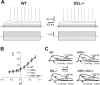
Intrinsic and synaptic properties of striatal spiny neurons of WT and D2 receptor mutants. A, The electrophysiological traces show that in WT and D2L-/- neurons, the membrane responses to hyperpolarizing and depolarizing current steps were similar. B, This graph shows that the current-voltage relationships of striatal neurons recorded from WT, D2R-/-, D2L-/-, and D2R-/-;D2L-/- mice were virtually coincident (n = 15 for each genotype). C, The electrophysiological traces are cortically evoked EPSPs recorded from WT, D2R-/-, D2L-/-, and D2R-/-;D2L-/- striatal neurons in the presence and in the absence of magnesium ions and in the presence of magnesium plus 10 μ
m
CNQX, antagonist of non-NMDA glutamate receptors. Note that in the absence of external magnesium, EPSP amplitude and duration increased in the four genotypes, as an expression of the contribution of glutamate NMDA receptors in corticostriatal transmission.
Figure 4.
DA-regulated synaptic plasticity in WT and mutant mice. A, The graph shows that HFS (arrow) of corticostriatal fibers induced LTD of glutamatergic transmission in WT neurons recorded in the presence of 1.2 m
m
external magnesium (LTD protocol). In contrast, the same stimulation protocol induces LTP in D2R-/-, D2L-/-, and D2R-/-;D2L-/- striatal neurons. Superimposed traces in the bottom part of the figure are intracellular recordings from single experiments showing EPSPs 10 min before (pre) and 30 min after (post) HFS in WT, D2R-/-, D2L-/-, and D2R-/-;D2L-/- striatal neurons. B, HFS delivered in the absence of magnesium ions from the bathing solution (LTP protocol) induced LTP in WT, D2R-/-, D2L-/-, and D2R-/-;D2L-/- striatal cells. Note that LTPs recorded from the three mutants had higher amplitude than the one recorded from WTs. C, The histogram shows that chronic haloperidol treatment enhanced the amplitude of corticostriatal LTP in WT mice but not in the three D2 receptor mutants. All these experiments were performed in the absence of magnesium ions. *p < 0.01 at 30 min after HFS. D, This graph shows that preincubation with the D1-like receptor antagonist SCH 23390 (10 μ
m
) prevented LTP in WT, D2R-/-, D2R-/-;D2L-/- neurons but not in D2L-/- cells. In this graph, LTP-inducing protocol in WT cells consisted in the application of HFS in the absence of magnesium ions. In contrast, in the three mutants, HFS was delivered either in the absence (at least 3 cells per group) or in the presence (at least 3 cells per group) of magnesium ions. Because the experiments gave similar results, the data were pooled together for each genotype.
Figure 5.
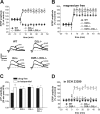
LTPs from haloperidol-treated rats and from D2 receptor mutant mice are sensitive to NMDA receptor and PKC blockade. A, B, The graphs show that preincubation with the glutamate NMDA receptor antagonist MK-801 (30 μ
m
) blocked LTP induction in control and chronic haloperidol-treated rats (A) as well as in WT and D2R-/-, D2L-/-, and D2R-/-;D2L-/- striatal neurons (B). Note that in control rats and in WT mice, blockade of NMDA receptors not only prevented LTP but also favored the emergence of LTD. C, D, Intracellular application of the PKC inhibitor RO 32-043 prevented LTP in control and chronic haloperidol-treated rats (C) as well as in WT and mutant mice (D). In the four graphs, the LTP-inducing protocol in WT cells consisted of the application of HFS in the absence of magnesium ions. In contrast, in the three mutants, HFS was delivered either in the absence (at least 4 cells per group and experimental condition) or in the presence (at least 4 cells per group and experimental condition) of magnesium ions. Because the experiments gave similar results, the data were pooled together for each genotype.
Figure 6.
Synaptic depotentiation is unaffected by chronic haloperidol treatment or genetic manipulations of D2 receptors. A, B, After the induction of LTP by HFS, a 10 min application of LFS (2 Hz) of corticostriatal terminals reversed this form of synaptic plasticity in control and chronic haloperidol-treated animals (A) as well as in WT and in the three genotypes of D2 receptor mutant mice. These experiments were performed in the absence of magnesium ions from the external solution because synaptic depotentiation is regulated by NMDA receptors.
Similar articles
- Distinct roles of D1 and D5 dopamine receptors in motor activity and striatal synaptic plasticity.
Centonze D, Grande C, Saulle E, Martin AB, Gubellini P, Pavón N, Pisani A, Bernardi G, Moratalla R, Calabresi P. Centonze D, et al. J Neurosci. 2003 Sep 17;23(24):8506-12. doi: 10.1523/JNEUROSCI.23-24-08506.2003. J Neurosci. 2003. PMID: 13679419 Free PMC article. - Differential contribution of dopamine D2S and D2L receptors in the modulation of glutamate and GABA transmission in the striatum.
Centonze D, Gubellini P, Usiello A, Rossi S, Tscherter A, Bracci E, Erbs E, Tognazzi N, Bernardi G, Pisani A, Calabresi P, Borrelli E. Centonze D, et al. Neuroscience. 2004;129(1):157-66. doi: 10.1016/j.neuroscience.2004.07.043. Neuroscience. 2004. PMID: 15489038 - The effects of dopamine and D2 receptor antagonists on pituitary hormone secretion are intact in mice lacking dopamine D2L receptor.
Xu R, Parlow AF, Wang Y. Xu R, et al. Brain Res. 2002 Jun 7;939(1-2):95-9. doi: 10.1016/s0006-8993(02)02559-3. Brain Res. 2002. PMID: 12020855 - Expression of N-methyl-D-aspartate receptor-dependent long-term potentiation in the neostriatal neurons in an in vitro slice after ethanol withdrawal of the rat.
Yamamoto Y, Nakanishi H, Takai N, Shimazoe T, Watanabe S, Kita H. Yamamoto Y, et al. Neuroscience. 1999;91(1):59-68. doi: 10.1016/s0306-4522(98)00611-3. Neuroscience. 1999. PMID: 10336060 - The Cdk5 inhibitor Roscovitine increases LTP induction in corticostriatal synapses.
Miranda-Barrientos J, Nieto-Mendoza E, Hernández-Echeagaray E. Miranda-Barrientos J, et al. ASN Neuro. 2014 Mar 19;6(2):e00140. doi: 10.1042/AN20140006. ASN Neuro. 2014. PMID: 24555476 Free PMC article.
Cited by
- Prenatal Stress Leads to the Altered Maturation of Corticostriatal Synaptic Plasticity and Related Behavioral Impairments Through Epigenetic Modifications of Dopamine D2 Receptor in Mice.
Li Y, Rong J, Zhong H, Liang M, Zhu C, Chang F, Zhou R. Li Y, et al. Mol Neurobiol. 2021 Jan;58(1):317-328. doi: 10.1007/s12035-020-02127-6. Epub 2020 Sep 15. Mol Neurobiol. 2021. PMID: 32935231 - YTHDF1 mediates translational control by m6A mRNA methylation in adaptation to environmental challenges.
Shi Z, Wen K, Zou Z, Fu W, Guo K, Sammudin NH, Ruan X, Sullere S, Wang S, Zhang X, Thinakaran G, He C, Zhuang X. Shi Z, et al. bioRxiv [Preprint]. 2024 Aug 9:2024.08.07.607063. doi: 10.1101/2024.08.07.607063. bioRxiv. 2024. PMID: 39149343 Free PMC article. Preprint. - The Emerging Role of Altered d-Aspartate Metabolism in Schizophrenia: New Insights From Preclinical Models and Human Studies.
Errico F, Nuzzo T, Carella M, Bertolino A, Usiello A. Errico F, et al. Front Psychiatry. 2018 Nov 6;9:559. doi: 10.3389/fpsyt.2018.00559. eCollection 2018. Front Psychiatry. 2018. PMID: 30459655 Free PMC article. Review. - Electrophysiological Endophenotypes for Schizophrenia.
Owens EM, Bachman P, Glahn DC, Bearden CE. Owens EM, et al. Harv Rev Psychiatry. 2016 Mar-Apr;24(2):129-47. doi: 10.1097/HRP.0000000000000110. Harv Rev Psychiatry. 2016. PMID: 26954597 Free PMC article. Review. - LRRK2 mediates haloperidol-induced changes in indirect pathway striatal projection neurons.
Chen C, Masotti M, Shepard N, Promes V, Tombesi G, Arango D, Manzoni C, Greggio E, Hilfiker S, Kozorovitskiy Y, Parisiadou L. Chen C, et al. Mol Psychiatry. 2025 Apr 23. doi: 10.1038/s41380-025-03030-z. Online ahead of print. Mol Psychiatry. 2025. PMID: 40269187
References
- Akopian G, Musleh W, Smith R, Walsh JP (2000) Functional state of corticostriatal synapses determines their expression of short- and long-term plasticity. Synapse 38: 271-280. - PubMed
- Baik J-H, Picetti R, Saiardi A, Thiriet G, Dierich A, Depaulis A, Le Meur M, Borrelli E (1995) Parkinsonian-like locomotor impairment in mice lacking dopamine D2 receptors. Nature 377: 424-428. - PubMed
- Bamford NS, Zhang H, Schmitz Y, Wu NP, Cepeda C, Levine MS, Schmauss C, Zakharenko SS, Zablow L, Sulzer D (2004) Heterosynaptic dopamine neurotransmission selects sets of corticostriatal terminals. Neuron 42: 653-663. - PubMed
- Bashir ZI, Collingridge GL (1994) An investigation of depotentiation of long-term potentiation in the CA1 region of the hippocampus. Exp Brain Res 100: 437-443. - PubMed
- Benes FM, Paskevich PA, Davidson J, Domesick VB (1985) The effects of haloperidol on synaptic patterns in the rat striatum. Brain Res 329: 265-273. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources