Cortical dysplasia resembling human type 2 lissencephaly in mice lacking all three APP family members - PubMed (original) (raw)
Comparative Study
. 2004 Oct 13;23(20):4106-15.
doi: 10.1038/sj.emboj.7600390. Epub 2004 Sep 23.
Affiliations
- PMID: 15385965
- PMCID: PMC524337
- DOI: 10.1038/sj.emboj.7600390
Comparative Study
Cortical dysplasia resembling human type 2 lissencephaly in mice lacking all three APP family members
Jochen Herms et al. EMBO J. 2004.
Abstract
The Alzheimer's disease beta-amyloid precursor protein (APP) is a member of a larger gene family that includes the amyloid precursor-like proteins, termed APLP1 and APLP2. We previously documented that APLP2-/-APLP1-/- and APLP2-/-APP-/- mice die postnatally, while APLP1-/-APP-/- mice and single mutants were viable. We now report that mice lacking all three APP/APLP family members survive through embryonic development, and die shortly after birth. In contrast to double-mutant animals with perinatal lethality, 81% of triple mutants showed cranial abnormalities. In 68% of triple mutants, we observed cortical dysplasias characterized by focal ectopic neuroblasts that had migrated through the basal lamina and pial membrane, a phenotype that resembles human type II lissencephaly. Moreover, at E18.5 triple mutants showed a partial loss of cortical Cajal Retzius (CR) cells, suggesting that APP/APLPs play a crucial role in the survival of CR cells and neuronal adhesion. Collectively, our data reveal an essential role for APP family members in normal brain development and early postnatal survival.
Figures
Figure 1
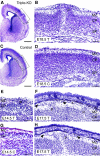
Cortical dysplasia in APP−/−APLP1−/−APLP2−/− triple knockout mice of various ages. (A, B) Frontal sections (cresyl violet staining) of a triple knockout mouse (T) at E18.5, exhibiting a prominent protrusion (P) of the right hemispheric cortical plate. The occurrence of ectopias was restricted to dorsal cortical areas as indicated by lines in (A). (B) Upon higher magnification (boxed area in A), it becomes apparent that ectopic neurons completely disrupt the subplate (SP) and cortical plate (CP); the cells have migrated into and beyond the MZ. No alterations were observed in the ventricular zone (not shown) (C, D) Depict low- and high-power images of a littermate control (C, genotype: APP−/−APLP1+/−APLP2−/−). (F) Example of an E17.5 triple knockout brain (T) showing two smaller cerebral protrusions (P). Arrowheads mark cells invading the subarachnoidal space (s). (E) Example of an E14.5 triple knockout exhibiting a small protrusion in comparison to a littermate control (C, genotype: APP−/−APLP1−/−APLP2+/−) depicted in (G). (H) Example of ectopia in a triple knockout embryo at E17.5. Note that a complete disruption of cortical layers is typically found within the center of dysplasias (B, H). In more lateral aspects (sections), the layering of deeper cortical structures appears much less affected (see, for example, F). Scale bars=500 μm (A, C), 100 μm (B, D, F, H), 50 μm (E, G).
Figure 2
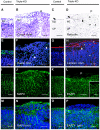
Immunohistochemical characterization of ectopias in APP−/−APLP1−/−APLP2−/− triple knockout mice at E18.5. (A, B) High-power views of cresyl violet-stained coronal sections of control (A) and (B) triple knockout cortex. (B) Shows a high power view of Figure 1B, depicting the border of a neuronal outgrowth with a well-defined MZ on the left and clustered ectopic neurons on the right (P, protrusion). (C, D) Staining of control (C) and triple knockout (D) sections for reticulin, a marker for collagen fibers in the basal lamina (BL) and pial layer revealed that the BL (arrow) was severely disrupted at the protrusion. Arrowheads indicate reticulin-stained thread-like abnormal structures. (E, F) CR cells in control (E) and triple mutant (F) sections stained for reelin (green, arrows). In controls and adjacent to ectopias, CR cells were properly located at the leptomeningeal side of the MZ. Note that, within ectopias, positioning of CR cells was severely disordered (as indicated by arrows) (G, H) Anti-laminin staining (red) of control (G) and triple mutant (H) sections. Triple mutants revealed normal laminin expression along the BL, which was, however, abrogated at the border neighboring the outgrowths (arrow). Aberrant punctate laminin staining is indicated by arrowheads. (K, L, O, P) Staining of radial glia (using an antibody against EAAT1, green) in the cerebral cortex of a control (K, O) and triple knockout. (L, P) Nuclei were stained with DAPI (see overlay depicted in P). (K, O) In control mice, radial glial fibers were found to extend through the cortical plate and MZ to the meningeal cell layer. (L) Within the protrusion, very little EAAT1 staining was detectable and fibers terminated prematurely. However, in unaffected regions of triple mutant cortex (inset in L depicts a high-power magnification), radial glial foot processes terminated normally at the pial–glial interface. (I, J, M, N) Immunostaining for MAPII (green) and counterstaining of nuclei with DAPI (blue) of control (I, M) and triple mutant (J, N) sections. Note that, within ectopias, MAPII staining is grossly disorganized. MZ, marginal zone; CP, cortical plate; scale bars=50 μm for all panels, except for the 15 μm scale bar within the inset in (L). Genotype of littermate controls: APP−/−APLP1+/−APLP2−/−.
Figure 3
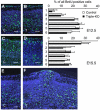
Neuronal migration in the cerebral cortex is unaltered in triple mutants. (A–F) BrdU birthdating analysis. Pregnant mice were injected with BrdU either at E12.5 or at E15.5 and the distribution of BrdU+ neurons (green) was determined at E18.5 and E19.5, respectively. (A, C) Sections of littermate controls (C) and (B, D) triple mutants (T) injected at E12.5 (A, B), or E15.5 (C, D). Panels on the right show a quantification of BrdU+ cells at various distances from the MZ. BrdU+ cells were counted in six stripes (consisting of five squares of 50 × 50 μm2) positioned underneath the MZ along the vertical axis. Note that statistical analysis (Student's _t_-test) did nor reveal any significant changes (_P_>0.05) in the distribution of BrdU+ neurons between triple knockouts (black bars) and littermate controls (open bars). The distribution of neurons labeled at E12.5 peaks within deeper layers of the cortical plate, whereas the majority of neurons labeled at E15.5 were found underneath the MZ. Values represent mean±s.d. of the percentage BrdU+ cells/stripe area relative to all positive cells from all six stripes obtained from four to five animals. (F) A high proportion of E12.5-injected BrdU+ cells are found throughout the protrusion of a triple knockout embryo sectioned at E18.5. (E) Unaffected littermate control for comparison. Genotypes of littermate controls: APP−/−APLP1−/−APLP2+/−. Scale bars=50 μm.
Figure 4
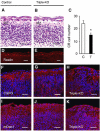
Alterations in the MZ of triple knockout mice. (A, B) Appearance of the MZ in HE-stained sections of (A) littermate control and (B) triple knockout cortices at E16.5. Note the prominent reduction in the number of nuclei in the MZ of triple mutants. (D, E) Immunohistochemistry for reelin (red) revealed a considerable reduction of CR cells in triple knockouts (E) as compared to controls (D) at E18.5. (C) The number of reelin-positive neurons within a 1200 μm wide MZ strip of the parietal cortex of E18.5 embryos was determined for both hemispheres on 8-μm-thick frozen frontal cortical sections. Values represent average cell counts±s.d. from _n_=8 stripes of four triple mutants (T, filled bar) and _n_=10 stripes of five littermate controls (C, open bar). *P<0.05, Student's _t_-test. (F–K) Frontal sections of E18.5 cortices from littermate control (F, I) and triple mutants (G, H, J, K) stained with antibodies against CSPG (F–H, red) or mDab1 (I–K, red). Cell nuclei were stained with DAPI (blue). The expression of CSPG and mDab1 within the MZ of triple knockouts (G, J) appeared unaffected in areas lacking protrusions. However, within protrusions (H, K), the normal pattern of expression of CSPG (H) and mDab1 (K) was interrupted and appeared disorganized. Scale bars=30 μm. Genotypes of littermate controls: APP−/−APLP1−/−APLP2+/− (A, D), APP−/−APLP1+/+APLP2+/+ (F), APP−/−APLP1+/+APLP2+/− (I).
Similar articles
- Mice with combined gene knock-outs reveal essential and partially redundant functions of amyloid precursor protein family members.
Heber S, Herms J, Gajic V, Hainfellner J, Aguzzi A, Rülicke T, von Kretzschmar H, von Koch C, Sisodia S, Tremml P, Lipp HP, Wolfer DP, Müller U. Heber S, et al. J Neurosci. 2000 Nov 1;20(21):7951-63. doi: 10.1523/JNEUROSCI.20-21-07951.2000. J Neurosci. 2000. PMID: 11050115 Free PMC article. - Differential role of APP and APLPs for neuromuscular synaptic morphology and function.
Klevanski M, Saar M, Baumkötter F, Weyer SW, Kins S, Müller UC. Klevanski M, et al. Mol Cell Neurosci. 2014 Jul;61:201-10. doi: 10.1016/j.mcn.2014.06.004. Epub 2014 Jul 4. Mol Cell Neurosci. 2014. PMID: 24998676 - APLP1 Is a Synaptic Cell Adhesion Molecule, Supporting Maintenance of Dendritic Spines and Basal Synaptic Transmission.
Schilling S, Mehr A, Ludewig S, Stephan J, Zimmermann M, August A, Strecker P, Korte M, Koo EH, Müller UC, Kins S, Eggert S. Schilling S, et al. J Neurosci. 2017 May 24;37(21):5345-5365. doi: 10.1523/JNEUROSCI.1875-16.2017. Epub 2017 Apr 27. J Neurosci. 2017. PMID: 28450540 Free PMC article. - The role of APP and APLP for synaptic transmission, plasticity, and network function: lessons from genetic mouse models.
Korte M, Herrmann U, Zhang X, Draguhn A. Korte M, et al. Exp Brain Res. 2012 Apr;217(3-4):435-40. doi: 10.1007/s00221-011-2894-6. Epub 2011 Oct 18. Exp Brain Res. 2012. PMID: 22006270 Review. - Redundancy and divergence in the amyloid precursor protein family.
Shariati SA, De Strooper B. Shariati SA, et al. FEBS Lett. 2013 Jun 27;587(13):2036-45. doi: 10.1016/j.febslet.2013.05.026. Epub 2013 May 23. FEBS Lett. 2013. PMID: 23707420 Review.
Cited by
- Interaction of the amyloid precursor protein-like protein 1 (APLP1) E2 domain with heparan sulfate involves two distinct binding modes.
Dahms SO, Mayer MC, Roeser D, Multhaup G, Than ME. Dahms SO, et al. Acta Crystallogr D Biol Crystallogr. 2015 Mar;71(Pt 3):494-504. doi: 10.1107/S1399004714027114. Epub 2015 Feb 26. Acta Crystallogr D Biol Crystallogr. 2015. PMID: 25760599 Free PMC article. - Deficits in axonal transport in hippocampal-based circuitry and the visual pathway in APP knock-out animals witnessed by manganese enhanced MRI.
Gallagher JJ, Zhang X, Ziomek GJ, Jacobs RE, Bearer EL. Gallagher JJ, et al. Neuroimage. 2012 Apr 15;60(3):1856-66. doi: 10.1016/j.neuroimage.2012.01.132. Epub 2012 Feb 10. Neuroimage. 2012. PMID: 22500926 Free PMC article. - Structural Studies Providing Insights into Production and Conformational Behavior of Amyloid-β Peptide Associated with Alzheimer's Disease Development.
Urban AS, Pavlov KV, Kamynina AV, Okhrimenko IS, Arseniev AS, Bocharov EV. Urban AS, et al. Molecules. 2021 May 13;26(10):2897. doi: 10.3390/molecules26102897. Molecules. 2021. PMID: 34068293 Free PMC article. Review. - The spinal muscular atrophy with pontocerebellar hypoplasia gene VRK1 regulates neuronal migration through an amyloid-β precursor protein-dependent mechanism.
Vinograd-Byk H, Sapir T, Cantarero L, Lazo PA, Zeligson S, Lev D, Lerman-Sagie T, Renbaum P, Reiner O, Levy-Lahad E. Vinograd-Byk H, et al. J Neurosci. 2015 Jan 21;35(3):936-42. doi: 10.1523/JNEUROSCI.1998-14.2015. J Neurosci. 2015. PMID: 25609612 Free PMC article. - Alzheimer disease models and human neuropathology: similarities and differences.
Duyckaerts C, Potier MC, Delatour B. Duyckaerts C, et al. Acta Neuropathol. 2008 Jan;115(1):5-38. doi: 10.1007/s00401-007-0312-8. Epub 2007 Nov 16. Acta Neuropathol. 2008. PMID: 18038275 Free PMC article. Review.
References
- Aherne W (1975) Some morphometric methods for the central nervous system. J Neurol Sci 24: 221–241 - PubMed
- Beher D, Hesse L, Masters CL, Multhaup G (1996) Regulation of amyloid protein precursor (APP) binding to collagen and mapping of the binding sites on APP and collagen type I. J Biol Chem 271: 1613–1620 - PubMed
- Blackshear PJ, Silver J, Nairn AC, Sulik KK, Squier MV, Stumpo DJ, Tuttle JS (1997) Widespread neuronal ectopia associated with secondary defects in cerebrocortical chondroitin sulfate proteoglycans and basal lamina in MARCKS-deficient mice. Exp Neurol 145: 46–61 - PubMed
- Cao X, Südhof TC (2001) A transcriptively active complex of APP with Fe65 and histone acetyltransferase Tip60. Science 293: 115–120 - PubMed
- Cao X, Südhof TC (2004) Dissection of amyloid b-precursor protein-dependent transcriptional activation. J Biol Chem 279: 2460–24611 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous