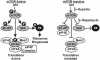Human cytomegalovirus infection induces rapamycin-insensitive phosphorylation of downstream effectors of mTOR kinase - PubMed (original) (raw)
Human cytomegalovirus infection induces rapamycin-insensitive phosphorylation of downstream effectors of mTOR kinase
Sagar B Kudchodkar et al. J Virol. 2004 Oct.
Abstract
Signaling mediated by the cellular kinase mammalian target of rapamycin (mTOR) activates cap-dependent translation under normal (nonstressed) conditions. However, translation is inhibited by cellular stress responses or rapamycin treatment, which inhibit mTOR kinase activity. We show that during human cytomegalovirus (HCMV) infection, viral protein synthesis and virus production proceed relatively normally when mTOR kinase activity is inhibited due to hypoxic stress or rapamycin treatment. Using rapamycin inhibition of mTOR, we show that HCMV infection induces phosphorylation of two mTOR effectors, eucaryotic initiation factor 4E (eIF4E) binding protein (4E-BP) and eIF4G. The virally induced phosphorylation of eIF4G is both mTOR and phosphatidylinositol 3-kinase (PI3K) independent, whereas the phosphorylation of 4E-BP is mTOR independent, but PI3K dependent. HCMV infection does not induce mTOR-independent phosphorylation of a third mTOR effector, p70S6 kinase (p70S6K). We show that the HCMV-induced phosphorylation of eIF4G and 4E-BP correlates with the association of eIF4E, the cap binding protein, with eIF4G in the eIF4F translation initiation complex. Thus, HCMV induces mechanisms to maintain the integrity of the eIF4F complex even when mTOR signaling is inhibited.
Figures
FIG. 1.
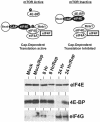
The mTOR signaling pathway. The phosphorylation characteristics of mTOR downstream effectors and the status of the eIF4F complex when mTOR is either active or inactive are shown. The points at which hypoxia and rapamycin inhibit mTOR are indicated. S6K, p70S6 kinase; Mnk1, mitogen-activated protein kinase signal-integrating kinase 1.
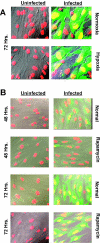
FIG. 2.
HCMV infects and spreads in LEHFF cells under hypoxic conditions and in the presence of rapamycin. (A) LEHFFs were mock infected (Uninfected) or infected with HCMV (Infected) for 72 h in the presence of serum under normoxic or hypoxic conditions. Cells were fixed and stained with DAPI (red) for nuclear visualization. The Towne strain of HCMV contains an inserted GFP gene which indicated the infected cells (green). The images were superimposed on differential interference contrast micrographs; overlap of the red and green shows the infected cell nuclei as yellow. (B) Serum-starved LEHFFs were mock or HCMV infected and maintained in serum-free medium with or without 50 nM rapamycin for 48 or 72 h. Microscopy was the same as for panel A.
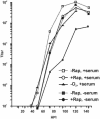
FIG. 3.
HCMV viral growth curves. LEHFFs were infected under normal conditions (−Rap, +serum), under hypoxic conditions (−O2, +serum), and in the presence of rapamycin (+Rap, +serum) and harvested at various times after infection up to 144 h. Virus titers at each time point were determined by the 50% tissue culture infective dose method. In addition, viral growth curves were generated under conditions where the cells were serum starved for 48 h and then infected and maintained in the absence of serum (−Rap, −serum) or in the absence of serum with the addition of rapamycin (+Rap, −serum).
FIG. 4.
The appearance and accumulation of HCMV immediate-early and early proteins occur with both hypoxia and rapamycin. (A) Cultures of LEHFFs were grown under normal serum conditions and mock infected (uninfected) or HCMV infected under normoxic (Norm) and hypoxic (Hyp) conditions for various times (hours) after infection (hpi). Extracts were analyzed by Western analysis for HCMV MIEP and delayed-early ICP36 (UL44) protein synthesis. (B) A similar experiment showing only the 48-h time point examines MIEP and ICP36 production and mTOR phosphorylation in the presence of rapamycin (Rap.) and hypoxia (Hypox.). The asterisk represents denatured viral MIEPs which result from harvesting after hypoxia treatment.
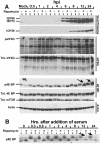
FIG. 5.
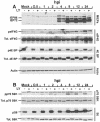
Effect of HCMV infection on total levels and phosphorylation status of mTOR effectors in the presence and absence of rapamycin. (A) Analysis of the MIEPs (IEP86 and IEP72), ICP36, eIF4G, 4E-BP, mTOR, and actin. LEHFFs were serum starved for 48 h and then mock or HCMV infected in serum-free medium with or without 50 nM rapamycin. Cells were extracted at various times after infection for up to 24 h, and the phosphorylation status (p) and/or total (Tot.) levels of various proteins were determined by Western analyses as described in Materials and Methods. Arrows mark the positions of hyperphosphorylated forms of 4E-BP generated in the presence of rapamycin. (B) Rapamycin inhibits serum-induced phosphorylation of 4E-BP over a 24-h time course. LEHFFs were serum starved for 48 h and then changed to medium containing 10% fetal calf serum with or without 50 nM rapamycin. At various times after the addition of serum, the phosphorylation status of 4E-BP was determined by Western analysis. Arrows indicate where hyperphosphorylated forms of 4E-BP should appear if they formed in the rapamycin-treated samples (compare with arrows in panel A).
FIG. 6.
HCMV infection reduces eIF4E binding to 4E-BP and increases its binding to eIF4G. LEHFFs were serum starved for 48 h and mock or HCMV infected in serum-free medium with or without 50 nM rapamycin (Rap) for 8 or 24 h. Extracts were incubated with 7Me-GTP-Sepharose beads to capture eIF4E and associated proteins as described in Materials and Methods. Western analysis was used to detect total eIF4E, 4E-BP, and eIF4G.
FIG. 7.
Analysis of p70S6K and its substrate ribosomal protein S6. The same extracts and conditions used for Fig. 5A were used to analyze the total levels and phosphorylation status of p70S6K and S6 protein.
FIG. 8.
Phosphorylation (activation) of Akt during a 24-h HCMV infection of LEHFFs. LEHFFs were serum starved for 48 h and then mock (M) or HCMV infected in serum-free medium with or without 50 nM rapamycin for up to 24 h. Extracts were analyzed for phosphorylated Akt (pAkt), total Akt, and actin. hpi, hours postinfection.
FIG. 9.
Effect of HCMV infection on total levels and phosphorylation status of mTOR effectors in the presence and absence of the PI3K inhibitor LY. LEHFFs were serum starved for 48 h and then mock or HCMV infected in serum-free medium with or without 50 μM LY. Cells were extracted at various times (hours) after infection (hpi) for up to 24 h, and the phosphorylation status (p) and/or total (Tot.) levels of various proteins were determined by Western analyses as described in Materials and Methods. (A) Analysis of the MIEPs (IEP86 and IEP72), eIF4G, 4E BP, and actin. (B) Analysis of p70S6K and its substrate ribosomal protein S6.
Similar articles
- Inhibition of mammalian target of rapamycin induces phosphatidylinositol 3-kinase-dependent and Mnk-mediated eukaryotic translation initiation factor 4E phosphorylation.
Wang X, Yue P, Chan CB, Ye K, Ueda T, Watanabe-Fukunaga R, Fukunaga R, Fu H, Khuri FR, Sun SY. Wang X, et al. Mol Cell Biol. 2007 Nov;27(21):7405-13. doi: 10.1128/MCB.00760-07. Epub 2007 Aug 27. Mol Cell Biol. 2007. PMID: 17724079 Free PMC article. - Human cytomegalovirus infection alters the substrate specificities and rapamycin sensitivities of raptor- and rictor-containing complexes.
Kudchodkar SB, Yu Y, Maguire TG, Alwine JC. Kudchodkar SB, et al. Proc Natl Acad Sci U S A. 2006 Sep 19;103(38):14182-7. doi: 10.1073/pnas.0605825103. Epub 2006 Sep 7. Proc Natl Acad Sci U S A. 2006. PMID: 16959881 Free PMC article. - Modulation of host cell stress responses by human cytomegalovirus.
Alwine JC. Alwine JC. Curr Top Microbiol Immunol. 2008;325:263-79. doi: 10.1007/978-3-540-77349-8_15. Curr Top Microbiol Immunol. 2008. PMID: 18637511 Review. - The molecular target of rapamycin (mTOR) as a therapeutic target against cancer.
Mita MM, Mita A, Rowinsky EK. Mita MM, et al. Cancer Biol Ther. 2003 Jul-Aug;2(4 Suppl 1):S169-77. Cancer Biol Ther. 2003. PMID: 14508096 Review.
Cited by
- Aberrant regulation of the Akt signaling network by human cytomegalovirus allows for targeting of infected monocytes.
Peppenelli MA, Miller MJ, Altman AM, Cojohari O, Chan GC. Peppenelli MA, et al. Antiviral Res. 2018 Oct;158:13-24. doi: 10.1016/j.antiviral.2018.07.015. Epub 2018 Jul 25. Antiviral Res. 2018. PMID: 30055197 Free PMC article. - Translational control of the abundance of cytoplasmic poly(A) binding protein in human cytomegalovirus-infected cells.
Perez C, McKinney C, Chulunbaatar U, Mohr I. Perez C, et al. J Virol. 2011 Jan;85(1):156-64. doi: 10.1128/JVI.01778-10. Epub 2010 Oct 27. J Virol. 2011. PMID: 20980505 Free PMC article. - Activation of the PI3K/Akt pathway early during vaccinia and cowpox virus infections is required for both host survival and viral replication.
Soares JA, Leite FG, Andrade LG, Torres AA, De Sousa LP, Barcelos LS, Teixeira MM, Ferreira PC, Kroon EG, Souto-Padrón T, Bonjardim CA. Soares JA, et al. J Virol. 2009 Jul;83(13):6883-99. doi: 10.1128/JVI.00245-09. Epub 2009 Apr 22. J Virol. 2009. PMID: 19386722 Free PMC article. - Human cytomegalovirus infection maintains mTOR activity and its perinuclear localization during amino acid deprivation.
Clippinger AJ, Maguire TG, Alwine JC. Clippinger AJ, et al. J Virol. 2011 Sep;85(18):9369-76. doi: 10.1128/JVI.05102-11. Epub 2011 Jul 6. J Virol. 2011. PMID: 21734039 Free PMC article. - A herpesvirus kinase that masquerades as Akt: you don't have to look like Akt, to act like it.
Chuluunbaatar U, Mohr I. Chuluunbaatar U, et al. Cell Cycle. 2011 Jul 1;10(13):2064-8. doi: 10.4161/cc.10.13.16242. Epub 2011 Jul 1. Cell Cycle. 2011. PMID: 21606676 Free PMC article.
References
- Arsham, A. M., J. J. Howell, and M. C. Simon. 2003. A novel hypoxia-inducible factor-independent hypoxic response regulating mammalian target of rapamycin and its targets. J. Biol. Chem. 278:29655-29660. - PubMed
- Arsham, A. M., D. R. Plas, C. B. Thompson, and M. C. Simon. 2002. PI3-K/Akt signaling is neither required for hypoxic stabilization of HIF-1 nor sufficient for HIF-1-dependent target gene transcription. J. Biol. Chem. 277:15162-15170. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous