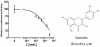Small molecules blocking the entry of severe acute respiratory syndrome coronavirus into host cells - PubMed (original) (raw)
. 2004 Oct;78(20):11334-9.
doi: 10.1128/JVI.78.20.11334-11339.2004.
Zhengquan Li, Kehu Yuan, Xiuxia Qu, Jian Chen, Guangwen Wang, Hong Zhang, Hongpeng Luo, Lili Zhu, Pengfei Jiang, Lirong Chen, Yan Shen, Min Luo, Guoying Zuo, Jianhe Hu, Deliang Duan, Yuchun Nie, Xuanling Shi, Wei Wang, Yang Han, Taisheng Li, Yuqing Liu, Mingxiao Ding, Hongkui Deng, Xiaojie Xu
Affiliations
- PMID: 15452254
- PMCID: PMC521800
- DOI: 10.1128/JVI.78.20.11334-11339.2004
Small molecules blocking the entry of severe acute respiratory syndrome coronavirus into host cells
Ling Yi et al. J Virol. 2004 Oct.
Abstract
Severe acute respiratory syndrome coronavirus (SARS-CoV) is the pathogen of SARS, which caused a global panic in 2003. We describe here the screening of Chinese herbal medicine-based, novel small molecules that bind avidly with the surface spike protein of SARS-CoV and thus can interfere with the entry of the virus to its host cells. We achieved this by using a two-step screening method consisting of frontal affinity chromatography-mass spectrometry coupled with a viral infection assay based on a human immunodeficiency virus (HIV)-luc/SARS pseudotyped virus. Two small molecules, tetra-O-galloyl-beta-D-glucose (TGG) and luteolin, were identified, whose anti-SARS-CoV activities were confirmed by using a wild-type SARS-CoV infection system. TGG exhibits prominent anti-SARS-CoV activity with a 50% effective concentration of 4.5 microM and a selective index of 240.0. The two-step screening method described here yielded several small molecules that can be used for developing new classes of anti-SARS-CoV drugs and is potentially useful for the high-throughput screening of drugs inhibiting the entry of HIV, hepatitis C virus, and other insidious viruses into their host cells.
Figures
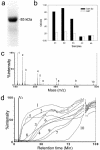
FIG. 1.
Identification of Galla chinensis components that have a high affinity to the SARS S2 protein by MS coupled with frontal affinity chromatography. (a) Purity of the GST-S fusion protein as shown by SDS-15% PAGE. (b) The specific binding of the GST-S2 protein with sera of three convalescent SARS patients (P1, P2, and P3) as shown by ELISA. Two normal sera (N1 and N2) and GST were used as controls. (c) The mass spectra of the crude extract of Galla chinensis (the main components are numbered 1 to 10; also see Table 1). (d) Frontal affinity chromatographic traces (selected ion chromatogram from the mass spectra, Fig. 1c) of the 10 main components in Galla chinensis. V0 indicates the volume of the nonbinding small molecules. Note the high retention time (_t_1/2 = 85 min) of component 10, indicating an exceptionally strong binding affinity to the SARS S2 protein.
FIG. 2.
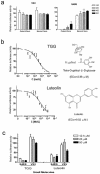
The inhibitory activities of SARS patients' sera and selected small molecules against the HIV-luc/SARS pseudotyped virus to enter Vero E6 cells. (a) Detection of inhibitory activities of sera of SARS patients. Note the ability of the SARS patient sera to block the infectivity of HIV-luc/SARS (SARS) versus the absence of such blocking activity in normal serum; note also the lack of neutralizing activity of the SARS sera against the pseudotyped virus bearing the G protein of VSV (VSV), which could infect the target cells at a similar level to that of HIV-luc/SARS. The serum dilution are indicated. (b) Structures and inhibitory activities of TGG and luteolin. Note the high inhibitory activity of TGG (EC50 = 2.86 μM), which is consistent with its high affinity to the SARS S2 protein, as our data demonstrated (Fig. 1c and d and Table 1). (c) Specificities of TGG and luteolin inhibitory activities. HIV-luc/VSV (VSV) pseudotyped viruses were used as a control. Note the inhibition by TGG and luteolin of the entry of HIV-luc/SARS virus but not the HIV-luc/VSV pseudotyped virus. Values are averages of triplicate determinations. The bars indicate the standard deviations.
FIG. 3.
Structure and inhibitory activities of quercetin against the entry of HIV-luc/SARS pseudotyped virus into Vero E6 cells. Similar results were obtained in three independent experiments.
Similar articles
- Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction.
Ho TY, Wu SL, Chen JC, Li CC, Hsiang CY. Ho TY, et al. Antiviral Res. 2007 May;74(2):92-101. doi: 10.1016/j.antiviral.2006.04.014. Epub 2006 May 15. Antiviral Res. 2007. PMID: 16730806 Free PMC article. - The anti-HIV drug nelfinavir mesylate (Viracept) is a potent inhibitor of cell fusion caused by the SARSCoV-2 spike (S) glycoprotein warranting further evaluation as an antiviral against COVID-19 infections.
Musarrat F, Chouljenko V, Dahal A, Nabi R, Chouljenko T, Jois SD, Kousoulas KG. Musarrat F, et al. J Med Virol. 2020 Oct;92(10):2087-2095. doi: 10.1002/jmv.25985. Epub 2020 May 17. J Med Virol. 2020. PMID: 32374457 Free PMC article. - Identification of a broad-spectrum antiviral small molecule against severe acute respiratory syndrome coronavirus and Ebola, Hendra, and Nipah viruses by using a novel high-throughput screening assay.
Elshabrawy HA, Fan J, Haddad CS, Ratia K, Broder CC, Caffrey M, Prabhakar BS. Elshabrawy HA, et al. J Virol. 2014 Apr;88(8):4353-65. doi: 10.1128/JVI.03050-13. Epub 2014 Feb 5. J Virol. 2014. PMID: 24501399 Free PMC article. - Severe acute respiratory syndrome coronavirus entry as a target of antiviral therapies.
Kuhn JH, Li W, Radoshitzky SR, Choe H, Farzan M. Kuhn JH, et al. Antivir Ther. 2007;12(4 Pt B):639-50. Antivir Ther. 2007. PMID: 17944271 Review. - Recent developments in the virology and antiviral research of severe acute respiratory syndrome coronavirus.
Yeung KS, Meanwell NA. Yeung KS, et al. Infect Disord Drug Targets. 2007 Mar;7(1):29-41. doi: 10.2174/187152607780090739. Infect Disord Drug Targets. 2007. PMID: 17346209 Review.
Cited by
- Plant Molecular Pharming and Plant-Derived Compounds towards Generation of Vaccines and Therapeutics against Coronaviruses.
Venkataraman S. Venkataraman S. Vaccines (Basel). 2022 Oct 26;10(11):1805. doi: 10.3390/vaccines10111805. Vaccines (Basel). 2022. PMID: 36366313 Free PMC article. Review. - Flavonoids as potential phytotherapeutics to combat cytokine storm in SARS-CoV-2.
Gour A, Manhas D, Bag S, Gorain B, Nandi U. Gour A, et al. Phytother Res. 2021 Aug;35(8):4258-4283. doi: 10.1002/ptr.7092. Epub 2021 Mar 30. Phytother Res. 2021. PMID: 33786876 Free PMC article. Review. - Phytochemicals containing biologically active polyphenols as an effective agent against Covid-19-inducing coronavirus.
Chojnacka K, Witek-Krowiak A, Skrzypczak D, Mikula K, Młynarz P. Chojnacka K, et al. J Funct Foods. 2020 Oct;73:104146. doi: 10.1016/j.jff.2020.104146. Epub 2020 Jul 30. J Funct Foods. 2020. PMID: 32834835 Free PMC article. Review. - A flavonoid, luteolin, cripples HIV-1 by abrogation of tat function.
Mehla R, Bivalkar-Mehla S, Chauhan A. Mehla R, et al. PLoS One. 2011;6(11):e27915. doi: 10.1371/journal.pone.0027915. Epub 2011 Nov 30. PLoS One. 2011. PMID: 22140483 Free PMC article. - Molecular and functional resemblance of dexamethasone and quercetin: A paradigm worth exploring in dexamethasone-nonresponsive COVID-19 patients.
Pawar A, Pal A. Pawar A, et al. Phytother Res. 2020 Dec;34(12):3085-3088. doi: 10.1002/ptr.6886. Epub 2020 Sep 30. Phytother Res. 2020. PMID: 33000510 Free PMC article. No abstract available.
References
- Baba, M., O. Nishimura, N. Kanzaki, M. Okamoto, H. Sawada, Y. Iizawa, M. Shiraishi, Y. Aramaki, K. Okonogi, Y. Ogawa, K. Meguro, and M. Fujino. 1999. A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc. Natl. Acad. Sci. USA 96:5698-5703. - PMC - PubMed
- Bartosch, B., J. Bukh, J. C. Meunie, C. Granier, R. E. Engle, W. C. Blackwelder, S. U. Emerson, F. L. Cosset, and R. H. Purcell. 2003. In vitro assay for neutralizing antibody to hepatitis C virus: evidence for broadly conserved neutralization epitopes. Proc. Natl. Acad. Sci. USA 100:14199-14204. - PMC - PubMed
- Bultmann, H., and C. R. Brandt. 2002. Peptides containing membrane-transiting motifs inhibit virus entry. J. Biol. Chem. 277:36018-36023. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous