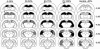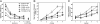Spatial memory, recognition memory, and the hippocampus - PubMed (original) (raw)
Spatial memory, recognition memory, and the hippocampus
Nicola J Broadbent et al. Proc Natl Acad Sci U S A. 2004.
Abstract
There is wide agreement that spatial memory is dependent on the integrity of the hippocampus, but the importance of the hippocampus for nonspatial tasks, including tasks of object recognition memory is not as clear. We examined the relationship between hippocampal lesion size and both spatial memory and object recognition memory in rats. Spatial memory was impaired after bilateral dorsal hippocampal lesions that encompassed 30-50% total volume, and as lesion size increased from 50% to approximately 100% of total hippocampal volume, performance was similarly impaired. In contrast, object recognition was intact after dorsal hippocampal lesions that damaged 50-75% of total hippocampal volume and was impaired only after larger lesions that encompassed 75-100% of hippocampal volume. Last, ventral hippocampal lesions that encompassed approximately 50% of total hippocampal volume impaired spatial memory but did not affect object recognition memory. These findings show that the hippocampus is important for both spatial memory and recognition memory. However, spatial memory performance requires more hippocampal tissue than does recognition memory.
Figures
Fig. 1.
Reconstructions of coronal sections through the hippocampus showing the smallest (black) and largest (stippled) lesion for each of the four hippocampal lesion groups (damage extending from the dorsal hippocampus to include 5-30%, 30-50%, 50-75%, and 75-100% of total hippocampal volume) from experiment 1 and the ventral lesion group (damage to ≈50% of total hippocampal volume) from experiment 2. Note that the locus and extent of hippocampal damage for the 50-75% and 75-100% groups were similar in experiments 1 and 2 (experiment 2 not shown). All rats sustained bilateral damage to the CA cell fields and dentate gyrus. In cases where the lesion was not complete at a particular level of the dorsal hippocampus, the sparing was typically restricted to the most medial aspect of the dentate gyrus or CA1 cell field. There was no evidence of damage to the amygdala or perirhinal cortex. Numbers (right) represent the distance (mm) posterior to bregma. For an additional description of the lesions, see Supporting Text.
Fig. 2.
Acquisition of spatial memory as a function of hippocampal lesion size. (A) Mean latency (sec) for the four dorsal hippocampal lesion groups (5-30%, 30-50%, 50-75%, and 75-100%) and the sham-operated group (SHAM) to find the platform during five daily training sessions (four training trials per day) and during visual platform training (two sessions, four trials per day). (B) Daily probe trial performance as measured by the percent time spent in the small training zone for the four dorsal hippocampal lesion groups and the sham-operated group. (C) Daily probe trial performance as measured by the percent time spent in the training quadrant for the four dorsal hippocampal groups and the sham-operated group. Probe trials were given at the beginning of each daily session before training (thus, probe trial 1 shows performance before platform training was begun). Brackets show the standard error of the mean.
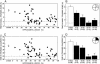
Fig. 3.
Performance on the spatial water-maze task as a function of hippocampal lesion size. Black circles and bars show performance of rats with hippocampal lesions. White circles and bars show performance of control rats (SHAM, n = 36). Brackets show the standard error of the mean. Hippocampal lesion group vs. SHAM group: *, P < 0.05; **, P < 0.01. (A) The scatter plot shows the percent time that the SHAM group and individual rats with hippocampal lesions spent in the small training zone averaged across probe trials that were given at the beginning of training sessions 2-5. (B) Percent time spent in the small training zone by the SHAM group and the hippocampal lesion groups (dorsal 5-30%, 30-50%, 50-75%, and 75-100% total hippocampal volume). The location of the small training zone in the water maze appears in the top right (black circle, diameter = 30 cm; chance performance = 4.0%). (C) The scatter plot shows the percent time that the SHAM group and individual rats with hippocampal lesions spent in the training quadrant averaged across probe trials 2-5. (D) Percent time spent in the training quadrant by the SHAM group and the hippocampal lesion groups. The location of the training quadrant in the water maze appears in the top right (black section; chance performance = 25.0%).
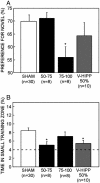
Fig. 4.
Performance of the two dorsal hippocampal groups (damage to 50-75% and 75-100% total hippocampal volume), the ventral hippocampal lesion group (V-HIPP 50%), and the SHAM group on the spatial water-maze task and the NOR task. (A) Preference for the novel object after a 3-h delay. Chance performance = 50%. (B) Percent time in the small training zone during probes 2-5. The broken line represents chance performance. Brackets show the standard error of the mean. *, Significantly different from SHAM.
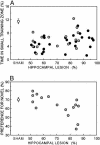
Fig. 5.
Scatter plots show performance on the spatial water-maze task (A) and the NOR task (B) as a function of lesion size (50-100% of total hippocampal volume). Black circles represent performance of individual rats with hippocampal lesions on the spatial water-maze task from experiment 1, and gray circles represent data from experiment 2. White circles show the mean performance of the SHAM group (n = 66 for the water maze, experiments 1 and 2 combined; n = 30 for NOR, experiment 2). Brackets show the standard error of the mean.
Similar articles
- Object recognition memory and the rodent hippocampus.
Broadbent NJ, Gaskin S, Squire LR, Clark RE. Broadbent NJ, et al. Learn Mem. 2009 Dec 22;17(1):5-11. doi: 10.1101/lm.1650110. Print 2010 Jan. Learn Mem. 2009. PMID: 20028732 Free PMC article. - When is the hippocampus involved in recognition memory?
Barker GR, Warburton EC. Barker GR, et al. J Neurosci. 2011 Jul 20;31(29):10721-31. doi: 10.1523/JNEUROSCI.6413-10.2011. J Neurosci. 2011. PMID: 21775615 Free PMC article. - Incidental (unreinforced) and reinforced spatial learning in rats with ventral and dorsal lesions of the hippocampus.
Gaskin S, Gamliel A, Tardif M, Cole E, Mumby DG. Gaskin S, et al. Behav Brain Res. 2009 Aug 24;202(1):64-70. doi: 10.1016/j.bbr.2009.03.016. Epub 2009 Mar 21. Behav Brain Res. 2009. PMID: 19447282 - Assessing rodent hippocampal involvement in the novel object recognition task. A review.
Cohen SJ, Stackman RW Jr. Cohen SJ, et al. Behav Brain Res. 2015 May 15;285:105-17. doi: 10.1016/j.bbr.2014.08.002. Epub 2014 Aug 26. Behav Brain Res. 2015. PMID: 25169255 Free PMC article. Review. - Hippocampus and neocortex: recognition and spatial memory.
Vann SD, Albasser MM. Vann SD, et al. Curr Opin Neurobiol. 2011 Jun;21(3):440-5. doi: 10.1016/j.conb.2011.02.002. Epub 2011 Feb 23. Curr Opin Neurobiol. 2011. PMID: 21353527 Review.
Cited by
- Assessing recollection and familiarity of similar lures in a behavioral pattern separation task.
Kim J, Yassa MA. Kim J, et al. Hippocampus. 2013 Apr;23(4):287-94. doi: 10.1002/hipo.22087. Epub 2013 Feb 8. Hippocampus. 2013. PMID: 23401187 Free PMC article. - Characterizing cognitive aging of recognition memory and related processes in animal models and in humans.
Burke SN, Ryan L, Barnes CA. Burke SN, et al. Front Aging Neurosci. 2012 Sep 12;4:15. doi: 10.3389/fnagi.2012.00015. eCollection 2012. Front Aging Neurosci. 2012. PMID: 22988437 Free PMC article. - High-THC Cannabis Smoke Impairs Incidental Memory Capacity in Spontaneous Tests of Novelty Preference for Objects and Odors in Male Rats.
Barnard IL, Onofrychuk TJ, Toderash AD, Patel VN, Glass AE, Adrian JC, Laprairie RB, Howland JG. Barnard IL, et al. eNeuro. 2023 Dec 11;10(12):ENEURO.0115-23.2023. doi: 10.1523/ENEURO.0115-23.2023. Print 2023 Dec. eNeuro. 2023. PMID: 37973381 Free PMC article. - Intravenous grafts of amniotic fluid-derived stem cells induce endogenous cell proliferation and attenuate behavioral deficits in ischemic stroke rats.
Tajiri N, Acosta S, Glover LE, Bickford PC, Jacotte Simancas A, Yasuhara T, Date I, Solomita MA, Antonucci I, Stuppia L, Kaneko Y, Borlongan CV. Tajiri N, et al. PLoS One. 2012;7(8):e43779. doi: 10.1371/journal.pone.0043779. Epub 2012 Aug 17. PLoS One. 2012. PMID: 22912905 Free PMC article. - Npas4: a neuronal transcription factor with a key role in social and cognitive functions relevant to developmental disorders.
Coutellier L, Beraki S, Ardestani PM, Saw NL, Shamloo M. Coutellier L, et al. PLoS One. 2012;7(9):e46604. doi: 10.1371/journal.pone.0046604. Epub 2012 Sep 28. PLoS One. 2012. PMID: 23029555 Free PMC article.
References
- Squire, L. R. (1992) Psychol. Rev. 99, 195-231. - PubMed
- Eichenbaum, H. & Cohen, N. J. (2001) From Conditioning to Conscious Recollection: Memory Systems of the Brain (Oxford Univ. Press, London).
- Suzuki, W. A. & Eichenbaum, H. (2000) Ann. N.Y. Acad. Sci. 911, 175-191. - PubMed
- Squire, L. R., Stark, C. E. L. & Clark, R. E. (2004) Annu. Rev. Neurosci. 27, 279-306. - PubMed
- Fujimichi, R., Naya, Y. & Miyashita, Y. in Cognitive Neurosciences III, ed. Gazzaniga, M. S. (MIT Press, Cambridge, MA), in press.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical