Spinal dorsal horn calcium channel alpha2delta-1 subunit upregulation contributes to peripheral nerve injury-induced tactile allodynia - PubMed (original) (raw)
Spinal dorsal horn calcium channel alpha2delta-1 subunit upregulation contributes to peripheral nerve injury-induced tactile allodynia
Chun-Ying Li et al. J Neurosci. 2004.
Abstract
Peripheral nerve injury induces upregulation of the calcium channel alpha2delta-1 structural subunit in dorsal root ganglia (DRG) and dorsal spinal cord of spinal nerve-ligated rats with neuropathic pain, suggesting a role of the calcium channel alpha2delta-1 subunit in central sensitization. To investigate whether spinal dorsal horn alpha2delta-1 subunit upregulation derives from increased DRG alpha2delta-1 subunit and plays a causal role in neuropathic pain development, we examined spinal dorsal hornalpha2delta-1 subunit expression with or without dorsal rhizotomy in spinal nerve-ligated rats and its correlation with tactile allodynia, a neuropathic pain state defined as reduced thresholds to non-noxious tactile stimulation. We also examined the effects of intrathecal alpha2delta-1 antisense oligonucleotides on alpha2delta-1 subunit expression and neuropathic allodynia in the nerve-ligated rats. Our data indicated that spinal nerve injury resulted in time-dependentalpha2delta-1 subunit upregulation in the spinal dorsal horn that correlated temporally with neuropathic allodynia development and maintenance. Dorsal rhizotomy diminished basal level expression and blocked injury-induced expression of the spinal dorsal hornalpha2delta-1 subunit and reversed injury-induced tactile allodynia. In addition, intrathecal alpha2delta-1 antisense oligonucleotides blocked injury-induced dorsal horn alpha2delta-1 subunit upregulation and diminished tactile allodynia. These findings indicate that alpha2delta-1 subunit basal expression occurs presynaptically and postsynaptically in spinal dorsal horn. Nerve injury induces mainly presynaptic alpha2delta-1 subunit expression that derives from increased alpha2delta-1 subunit in injured DRG neurons. Thus, changes in presynaptic alpha2delta-1 subunit expression contribute to injury-induced spinal neuroplasticity and central sensitization that underlies neuropathic pain development and maintenance.
Figures
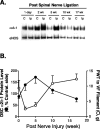
Figure 1.
Injury-induced upregulation of spinal dorsal horn α2δ-1 subunit correlated with the development and maintenance of neuropathic allodynia. Unilateral L5/L6 spinal nerve ligation surgeries were performed, PWTs to von Frey filament stimulation were measured, and tissue samples were collected for Western blot analysis at indicated time points after nerve injury. A, Representative Western blots (from n = 4) showing time-dependent upregulation of the α2δ-1 subunit in spinal dorsal horn ipsilateral to spinal nerve ligation injury. The same blots were striped and reblotted with anti-eNOS antibodies for normalization of loading. B, Summarized injury-induced α2δ-1 subunit upregulation in dorsal spinal cord (DSC; as shown in A) and its reciprocal relationship with the development of tactile allodynia indicated as reduced PWTs to von Frey (VF) filament stimulation. Band density ratios of α2δ-1 subunit over eNOS were taken before signals from the ipsilateral (injury) side were compared with those from the contralateral (noninjury) side, which were taken as 100%. Normal PWT in sham-operated and naive animals are between 10 and 15 gm (Luo et al., 2001; Valder et al., 2003). Mean ± SEM from four rats in each group. C, Contralateral side; Ip, ipsilateral side.
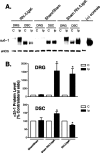
Figure 2.
Effects of rhizotomy onα2δ-1 subunit expression in dorsal spinal cord and DRG of sham and nerve-injured rats. Rhizotomy (Rhi.) and spinal nerve ligation (Ligat.) surgeries were performed at the same time, and tissue samples were collected for Western blot analyses 1 week after the surgeries. A shows a representative Western blot (from n = 5) indicating α2δ-1 protein levels in DRG and dorsal spinal cord (DSC) of neuropathic rats with or without dorsal rhizotomy. The same blots were stripped and reblotted with antibodies against eNOS for determination of equal loading. B shows summarized α2δ-1 protein levels in DRG and dorsal spinal cord detected with Western blots shown in A. Data shown are means ± SEM of percentage changes compared with that in contralateral sides (chosen as 100%) from at least five independent animals in each group. The asterisk indicates significant changes compared with the control values (p < 0.05) as determined by unpaired two-tailed Student's t test. C, Contralateral side; Ip, ipsilateral side; (+), positive.
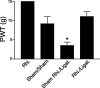
Figure 3.
Effects of rhizotomy on spinal nerve ligation induced tactile allodynia in rats. Rhizotomy (Rhi.) and spinal nerve ligation (Ligat.) surgeries were performed at the same time. PWTs to von Frey filament stimulation were measured in the injured paws 1 week after surgery and reported as means ± SEM from five independent animals in each group. In rhizotomized animals, the PWT to similar stimulation is 15 gm, similar to that in sham-operated and naive rats (between 10 and 15 gm) (Luo et al., 2001). The asterisk indicates significant changes (p < 0.05) compared with the sham control values determined by unpaired two-tailed Student's t test.
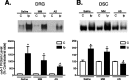
Figure 4.
Effects of intrathecal treatments with α2δ-1 antisense oligonucleotides on α2δ-1 subunit protein levels in dorsal spinal cord and DRG of nerve-injured rats. Spinal nerve ligation surgeries were performed 2 weeks before intrathecal catheterization. Thirty micrograms per rat of antisense (AS) or mismatch (MM) oligonucleotides in 10 μl of sterile saline, or 10 μl of sterile saline alone, followed by a 10 μl sterile saline flush were administered twice daily for 4 d through the intrathecal catheter at least 1 week after intrathecal catheterization. Western blot analyses were performed to detect the α2δ-1 protein levels in DRG (A) and dorsal spinal cord (DSC; B). The same plots were stripped and reblotted with antibodies against either eNOS or GAPDH for loading controls. Band density ratios of the α2δ-1 subunit over the eNOS or GAPDH controls were taken before signals from the ipsilateral (injury) side were compared with those from the contralateral (noninjury) side, which were taken as 100%. Summarized data were reported as means ± SEM from three to five independent animals examined in duplicate. The asterisk indicates significant changes (p < 0.05) compared with values from the contralateral side determined by paired two-tailed Student's t test. The # symbol indicates significant changes (p < 0.05) compared with values from saline-treated samples ipsilateral to the injury determined by unpaired two-tailed Student's t test. C, Contralateral side; Ip, ipsilateral side.
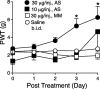
Figure 5.
Effects of intrathecal treatments with α2δ-1 antisense oligonucleotides on tactile allodynia of nerve-injured rats. Spinal nerve ligation surgeries were performed 2 weeks before intrathecal catheterization. Antisense (AS) or mismatch (MM) oligonucleotides in 10 μl of sterile saline, or 10 μl of sterile saline alone, followed by a 10 μl sterile saline flush were administered twice daily for 4 d through the intrathecal catheter at least 1 week after intrathecal catheterization. PWTs in the injury side to von Frey filament stimulation were measured before the intrathecal treatment and daily before the first injection for 4 d and reported as means ± SEM from at least six animals in each group. The asterisk indicates significant changes (p < 0.05) compared with values from saline-treated animals determined by unpaired two-tailed Student's t test.
Similar articles
- Calcium channel alpha-2-delta-1 protein upregulation in dorsal spinal cord mediates spinal cord injury-induced neuropathic pain states.
Boroujerdi A, Zeng J, Sharp K, Kim D, Steward O, Luo DZ. Boroujerdi A, et al. Pain. 2011 Mar;152(3):649-655. doi: 10.1016/j.pain.2010.12.014. Epub 2011 Jan 15. Pain. 2011. PMID: 21239111 Free PMC article. - Upregulation of calcium channel alpha-2-delta-1 subunit in dorsal horn contributes to spinal cord injury-induced tactile allodynia.
Kusuyama K, Tachibana T, Yamanaka H, Okubo M, Yoshiya S, Noguchi K. Kusuyama K, et al. Spine J. 2018 Jun;18(6):1062-1069. doi: 10.1016/j.spinee.2018.01.010. Epub 2018 Jan 31. Spine J. 2018. PMID: 29355786 - Upregulation of dorsal root ganglion (alpha)2(delta) calcium channel subunit and its correlation with allodynia in spinal nerve-injured rats.
Luo ZD, Chaplan SR, Higuera ES, Sorkin LS, Stauderman KA, Williams ME, Yaksh TL. Luo ZD, et al. J Neurosci. 2001 Mar 15;21(6):1868-75. doi: 10.1523/JNEUROSCI.21-06-01868.2001. J Neurosci. 2001. PMID: 11245671 Free PMC article. - Presynaptic NMDA receptors control nociceptive transmission at the spinal cord level in neuropathic pain.
Deng M, Chen SR, Pan HL. Deng M, et al. Cell Mol Life Sci. 2019 May;76(10):1889-1899. doi: 10.1007/s00018-019-03047-y. Epub 2019 Feb 20. Cell Mol Life Sci. 2019. PMID: 30788514 Free PMC article. Review. - Role of the immune system in neuropathic pain.
Malcangio M. Malcangio M. Scand J Pain. 2019 Dec 18;20(1):33-37. doi: 10.1515/sjpain-2019-0138. Scand J Pain. 2019. PMID: 31730538 Review.
Cited by
- Calcium Signalling in Breast Cancer Associated Bone Pain.
Bortolin A, Neto E, Lamghari M. Bortolin A, et al. Int J Mol Sci. 2022 Feb 8;23(3):1902. doi: 10.3390/ijms23031902. Int J Mol Sci. 2022. PMID: 35163823 Free PMC article. Review. - Silencing of the Cav3.2 T-type calcium channel gene in sensory neurons demonstrates its major role in nociception.
Bourinet E, Alloui A, Monteil A, Barrère C, Couette B, Poirot O, Pages A, McRory J, Snutch TP, Eschalier A, Nargeot J. Bourinet E, et al. EMBO J. 2005 Jan 26;24(2):315-24. doi: 10.1038/sj.emboj.7600515. Epub 2004 Dec 16. EMBO J. 2005. PMID: 15616581 Free PMC article. - Regulation of synaptic connectivity by glia.
Eroglu C, Barres BA. Eroglu C, et al. Nature. 2010 Nov 11;468(7321):223-31. doi: 10.1038/nature09612. Nature. 2010. PMID: 21068831 Free PMC article. Review. - Autophagy impairment in a mouse model of neuropathic pain.
Berliocchi L, Russo R, Maiarù M, Levato A, Bagetta G, Corasaniti MT. Berliocchi L, et al. Mol Pain. 2011 Oct 24;7:83. doi: 10.1186/1744-8069-7-83. Mol Pain. 2011. PMID: 22023914 Free PMC article. - Repetitive transcranial magnetic stimulation in central post-stroke pain: current status and future perspective.
Radiansyah RS, Hadi DW. Radiansyah RS, et al. Korean J Pain. 2023 Oct 1;36(4):408-424. doi: 10.3344/kjp.23220. Korean J Pain. 2023. PMID: 37752663 Free PMC article. Review.
References
- Ahlijanian MK, Westenbroek RE, Catterall WA (1990) Subunit structure and localization of dihydropyridine-sensitive calcium channels in mammalian brain, spinal cord, and retina. Neuron 4: 819-832. - PubMed
- Brust PF, Simerson S, McCue AF, Deal CR, Schoonmaker S, Williams ME, Veliçelebi G, Johnson EC, Harpold MM, Ellis SB (1993) Human neuronal voltage-dependent calcium channels: studies on subunit structure and role in channel assembly. Neuropharmacology 32: 1089-1102. - PubMed
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53: 55-63. - PubMed
- Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sik A, De Koninck P, De Koninck Y (2003) Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature 424: 938-942. - PubMed
- De Jongh KS, Warner C, Catterall WA (1990) Subunits of purified calcium channels. α2 and δ are encoded by the same gene. J Biol Chem 265: 14738-14741. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS40135/NS/NINDS NIH HHS/United States
- DE14545/DE/NIDCR NIH HHS/United States
- DE13270/DE/NIDCR NIH HHS/United States
- R01 NS040135/NS/NINDS NIH HHS/United States
- R21 DE014545/DE/NIDCR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical