Comparison of ABF1 and RAP1 in chromatin opening and transactivator potentiation in the budding yeast Saccharomyces cerevisiae - PubMed (original) (raw)
Comparative Study
Comparison of ABF1 and RAP1 in chromatin opening and transactivator potentiation in the budding yeast Saccharomyces cerevisiae
Arunadevi Yarragudi et al. Mol Cell Biol. 2004 Oct.
Abstract
Autonomously replicating sequence binding factor 1 (ABF1) and repressor/activator protein 1 (RAP1) from budding yeast are multifunctional, site-specific DNA-binding proteins, with roles in gene activation and repression, replication, and telomere structure and function. Previously we have shown that RAP1 can prevent nucleosome positioning in the vicinity of its binding site and have provided evidence that this ability to create a local region of "open" chromatin contributes to RAP1 function at the HIS4 promoter by facilitating binding and activation by GCN4. Here we examine and directly compare to that of RAP1 the ability of ABF1 to create a region of open chromatin near its binding site and to contribute to activated transcription at the HIS4, ADE5,7, and HIS7 promoters. ABF1 behaves similarly to RAP1 in these assays, but it shows some subtle differences from RAP1 in the character of the open chromatin region near its binding site. Furthermore, although the two factors can similarly enhance activated transcription at the promoters tested, RAP1 binding is continuously required for this enhancement, but ABF1 binding is not. These results indicate that ABF1 and RAP1 achieve functional similarity in part via mechanistically distinct pathways.
Figures
FIG. 1.
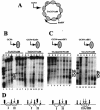
ABF1 perturbs nucleosome positioning via a nucleosomal binding site similarly to RAP1. (A) Schematic diagram of plasmid TA/GCN1Δ80. The gray ellipses represent positioned nucleosomes, and nucleosomes I and II are marked. Nucleosome I contains the GCN4 binding site, as shown, and chromatin structure was mapped clockwise from the EcoRV site, as indicated, by use of chromatin prepared from FY24 yeast cells containing the indicated plasmid episome. (B) Indirect end-label analysis of TA/GCN1Δ80 and TAR/GCN1Δ80 chromatin structure. TAR/GCN1Δ80 is similar to TA/GCN1Δ80 except for a RAP1 binding site introduced adjacent to the GCN4 binding site. MNase cleavage sites were mapped clockwise from the EcoRV site in naked DNA (D lanes) or in chromatin (C lanes) from cells grown in glucose medium under conditions that are noninducing for Gcn4p activation. Lane 1 contains φX/HaeIII marker DNA. Naked DNA in TA/GCN1Δ80 (lanes 2 to 4) and in TAR/GCN1Δ80 (lanes 8, 10, and 11) was digested using 2.5 (lanes 2 and 8), 5 (lanes 3 and 10), or 10 (lane 4 and 11) U of MNase per ml. Chromatin was digested using 0 (lane 9), 2.5 (lanes 5 and 12), 5 (lanes 6 and 13), or 10 (lanes 7 and 14) U/ml. (C) Indirect end-label analysis of TAA/GCN1Δ80 and TAAmut/GCN1Δ80 chromatin structure. TAA/GCN1Δ80 and TAAmut/GCN1Δ80 are similar to TA/GCN1Δ80, except for ABF1 or mutated ABF1 binding sites introduced adjacent to the GCN4 binding site. MNase cleavage sites were mapped clockwise from the EcoRV site in naked DNA (D lanes) or in chromatin (C lanes) from cells grown in glucose medium. Lane 9 contains φX/HaeIII marker DNA. Naked TAA/GCN1Δ80 and TAAmut/GCN1Δ80 DNA were digested using 2.5 (lanes 1 and 10), 5 (lanes 2 and 12), or 10 (lane 3) U/ml. Chromatin was digested using 0 (lane 11), 2.5 (lanes 4 and 13), 5 (lanes 5 and 14), 10 (lanes 6 and 15), 15 (lane 7), or 20 (lane 8) U of MNase per ml. All the chromatin samples were run on the same gel; the DNA lanes are derived from separate gels (Fig. 3 shows examples in which chromatin and naked DNA samples all derive from the same gel). The asterisks and open circles indicate cleavages in naked DNA that are protected by nucleosomes I and II in chromatin, and filled circles indicate the edges of nucleosomes I and II, which are present in TA/GCN1Δ80 and TAAmut/GCN1Δ80 but not in TAR/GCN1Δ80 and TAA/GCN1Δ80. The locations of positioned nucleosomes I and II are shown by ellipses. (D) Schematic diagram of nucleosome positioning in the region of nucleosomes I and II deduced from the MNase cutting patterns of TA/GCN1Δ80, TAR/GCN1Δ80, TAA/GCN1Δ80, and TAAmut/GCN1Δ80. The thickness of each vertical arrow indicates the relative strength of MNase cleavage.
FIG. 2.
Schematic diagram of ABF1. (A) Full-length ABF1 is shown, with DNA-binding domains, transactivation domain, and the C-terminal sequences (CS1 and CS2) indicated. (B) The truncated versions of ABF1 used in this work are shown.
FIG. 3.
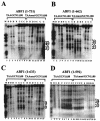
Contribution of C-terminal domains of ABF1 to chromatin perturbation via a nucleosomal binding site. (A) MNase cleavage sites in plasmids TAA/GCN1Δ80 and TAAmut/GCN1Δ80 were mapped in GCN4+ yeast harboring ABF1(1-731). Cleavage sites were mapped in naked DNA (D lanes) or in chromatin (C lanes) from the EcoRV site, as in Fig. 1. Lane 1 contains φX/HaeIII marker DNA. Locations of positioned nucleosomes I and II are indicated by ellipses. The filled circles to the right of the panel indicate cleavages enhanced in chromatin relative to DNA, and the stars indicate strong cleavages in naked DNA that are protected by nucleosomes I and II in chromatin of TAAmut/GCN1Δ80 but not TAA/GCN1Δ80. Each lane, beginning with lanes 2 to 4, differs only in the concentration of MNase used. Lanes 5 and 12 show controls not treated with MNase. (B) MNase cleavage sites in plasmids TAA/GCN1Δ80 and TAAmut/GCN1Δ80 were mapped in yeast harboring ABF1(1-662), as in panel A. (C) MNase cleavage sites in plasmids TAA/GCN1Δ80 and TAAmut/GCN1Δ80 were mapped in yeast harboring ABF1(1-633) as in panel A. (D) MNase cleavage sites in plasmids TAA/GCN1Δ80 and TAAmut/GCN1Δ80 were mapped in yeast harboring ABF1(1-592) as in panel A. All samples were run on the same gel, but some lanes are omitted from the figure, as seen from the visible “splice” marks.
FIG. 4.
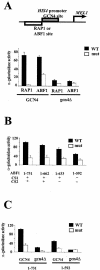
ABF1 can contribute to activation of the HIS4 promoter similarly to RAP1. (A) The MEL1 reporter gene fused to modified HIS4 promoter having an ABF1 or RAP1 binding site or mutant ABF1 or RAP1 binding sites adjacent to the GCN4 binding site, schematized at the top. MEL1 activity was monitored in GCN4+ cells or _gcn4_Δ cells and expressed as α-galactosidase activity. (B) Contribution of C-terminal domains to GCN4-mediated activation of the HIS4 promoter. The modified HIS4 promoter having an ABF1 binding site or mutant ABF1 binding site adjacent to the GCN4 binding site was introduced into yeast expressing ABF1 deletion mutants as indicated, and MEL1 activity was monitored. (C) MEL1 activity was monitored from the modified HIS4 promoter having an ABF1 binding site or mutant ABF1 binding site adjacent to the GCN4 binding site in GCN4+ or _gcn4_Δ yeast harboring full-length ABF1 (“1-731”) or truncated ABF1 lacking the CS1 and CS2 domains (“1-592”), as indicated. Standard deviations are indicated. WT, wild type.
FIG. 5.
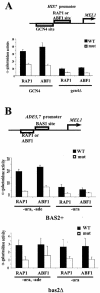
Comparison of ABF1 and RAP1 in facilitating transcriptional activation from the HIS7 and ADE5,7 promoters. (A) The MEL1 reporter gene fused to a modified HIS7 promoter having an ABF1 binding site or RAP1 binding site or mutant ABF1 or RAP1 site adjacent to the GCN4 binding site, schematized at the top. MEL1 activity was monitored and compared in GCN4+ wild-type cells and in _gcn4_Δ cells. Standard deviations are shown. (B) The MEL1 reporter gene fused to a modified ADE5,7 promoter having an ABF1 binding site or RAP1 binding site or mutant ABF1 or RAP1 binding site adjacent to the BAS1 binding site, schematized at the top. _MEL1_activity was monitored and compared in BAS1 and BAS2+ wild-type cells and in _bas2_Δ cells (note different scales) in the presence and absence of adenine. Standard deviations are shown. WT, wild type.
FIG. 6.
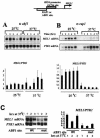
Continuous ABF1 binding to DNA is not required for efficient GCN4-mediated HIS4 activation. RNA was harvested from abf1 ts (A) and rap1-2 ts (B) cells harboring the HIS4-MEL1 reporter (schematized at the top) with either an ABF1 binding site (A) or a RAP1 binding site (B) after growth for the indicated times, in hours, at either 25 or 37°C. The RNA blots were hybridized with probes specific for MEL1 and PYK1 mRNA, and the signals were quantitated by PhosphorImager analysis. The bar graphs at the bottom show values derived from four independent experiments. Values for each experiment were normalized to the average MEL1/PYK1 value obtained for all time points at 25°C, and standard deviations are indicated. (C) Comparison of MEL1 mRNA levels (normalized to those of PYK1) from the modified HIS4 promoter having a wild-type or mutant ABF1 site, as indicated, after 1 to 3 h of growth at 37°C. All six lanes (for each mRNA) derive from a single exposure of the same blot. Quantitations in the bar graph on the right are from three independent experiments and were scaled by setting the average MEL1-PYK1 value for all three time points for the promoter having the wild-type ABF1 site to 1.
FIG. 7.
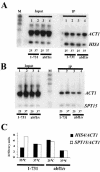
ChIP analysis of ABF1 binding to the modified HIS4-MEL1 promoter. ChIP was performed with ABF1 antibody in yeast carrying ABF1(1-731) or the abf1-1 ts mutant at 25°C or after 1 h at 37°C, as indicated. Input and IP samples were amplified in separate reactions with the use of primers for the HIS4-MEL1, SPT15, and ACT1 promoters. Samples were combined prior to electrophoresis, Southern blotting, and hybridization with appropriate probes. Although ACT1 was used as a negative control, its amplification was more efficient than that of HIS4-MEL1 or SPT15 promoters, for unknown reasons. Note, however, that ACT1 shows reduction in the IP lanes relative to the input lanes, and HIS4-MEL1 and SPT15 do not. Ratios of HIS4-MEL1 IP samples to input samples and SPT15 IP samples to input samples, relative to the ratio of ACT1 IP samples to input samples, are shown at the bottom. Similar results were obtained in this and one other independent experiment.
Similar articles
- Genome-wide analysis of transcriptional dependence and probable target sites for Abf1 and Rap1 in Saccharomyces cerevisiae.
Yarragudi A, Parfrey LW, Morse RH. Yarragudi A, et al. Nucleic Acids Res. 2007;35(1):193-202. doi: 10.1093/nar/gkl1059. Epub 2006 Dec 7. Nucleic Acids Res. 2007. PMID: 17158163 Free PMC article. - The N-terminal and C-terminal domains of RAP1 are dispensable for chromatin opening and GCN4-mediated HIS4 activation in budding yeast.
Yu L, Sabet N, Chambers A, Morse RH. Yu L, et al. J Biol Chem. 2001 Aug 31;276(35):33257-64. doi: 10.1074/jbc.M104354200. Epub 2001 Jun 18. J Biol Chem. 2001. PMID: 11413146 - Chromatin opening and transactivator potentiation by RAP1 in Saccharomyces cerevisiae.
Yu L, Morse RH. Yu L, et al. Mol Cell Biol. 1999 Aug;19(8):5279-88. doi: 10.1128/MCB.19.8.5279. Mol Cell Biol. 1999. PMID: 10409719 Free PMC article. - Multiple roles of the general regulatory factor Abf1 in yeast ribosome biogenesis.
Fermi B, Bosio MC, Dieci G. Fermi B, et al. Curr Genet. 2017 Feb;63(1):65-68. doi: 10.1007/s00294-016-0621-3. Epub 2016 Jun 4. Curr Genet. 2017. PMID: 27262581 Review. - The different (sur)faces of Rap1p.
Piña B, Fernández-Larrea J, García-Reyero N, Idrissi FZ. Piña B, et al. Mol Genet Genomics. 2003 Mar;268(6):791-8. doi: 10.1007/s00438-002-0801-3. Epub 2003 Jan 25. Mol Genet Genomics. 2003. PMID: 12655405 Review.
Cited by
- Shelterin Complex at Telomeres: Implications in Ageing.
Mir SM, Samavarchi Tehrani S, Goodarzi G, Jamalpoor Z, Asadi J, Khelghati N, Qujeq D, Maniati M. Mir SM, et al. Clin Interv Aging. 2020 Jun 3;15:827-839. doi: 10.2147/CIA.S256425. eCollection 2020. Clin Interv Aging. 2020. PMID: 32581523 Free PMC article. Review. - Genome-wide analysis of transcriptional dependence and probable target sites for Abf1 and Rap1 in Saccharomyces cerevisiae.
Yarragudi A, Parfrey LW, Morse RH. Yarragudi A, et al. Nucleic Acids Res. 2007;35(1):193-202. doi: 10.1093/nar/gkl1059. Epub 2006 Dec 7. Nucleic Acids Res. 2007. PMID: 17158163 Free PMC article. - Central role of Ifh1p-Fhl1p interaction in the synthesis of yeast ribosomal proteins.
Rudra D, Zhao Y, Warner JR. Rudra D, et al. EMBO J. 2005 Feb 9;24(3):533-42. doi: 10.1038/sj.emboj.7600553. Epub 2005 Feb 3. EMBO J. 2005. PMID: 15692568 Free PMC article. - Genome-wide determinants of sequence-specific DNA binding of general regulatory factors.
Rossi MJ, Lai WKM, Pugh BF. Rossi MJ, et al. Genome Res. 2018 Apr;28(4):497-508. doi: 10.1101/gr.229518.117. Epub 2018 Mar 21. Genome Res. 2018. PMID: 29563167 Free PMC article. - Genome-wide off-rates reveal how DNA binding dynamics shape transcription factor function.
de Jonge WJ, Brok M, Lijnzaad P, Kemmeren P, Holstege FC. de Jonge WJ, et al. Mol Syst Biol. 2020 Oct;16(10):e9885. doi: 10.15252/msb.20209885. Mol Syst Biol. 2020. PMID: 33280256 Free PMC article.
References
- Chasman, D. I., N. F. Lue, A. R. Buchman, J. W. LaPointe, Y. Lorch, and R. D. Kornberg. 1990. A yeast protein that influences the chromatin structure of UASG and functions as a powerful auxiliary gene activator. Genes Dev. 4:503-514. - PubMed
- Diffley, J. F., and B. Stillman. 1989. Similarity between the transcriptional silencer binding proteins ABF1 and RAP1. Science 246:1034-1038. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials