Mutational analysis of the N-terminal DNA-binding domain of sleeping beauty transposase: critical residues for DNA binding and hyperactivity in mammalian cells - PubMed (original) (raw)
Mutational analysis of the N-terminal DNA-binding domain of sleeping beauty transposase: critical residues for DNA binding and hyperactivity in mammalian cells
Stephen R Yant et al. Mol Cell Biol. 2004 Oct.
Abstract
The N-terminal domain of the Sleeping Beauty (SB) transposase mediates transposon DNA binding, subunit multimerization, and nuclear translocation in vertebrate cells. For this report, we studied the relative contributions of 95 different residues within this multifunctional domain by large-scale mutational analysis. We found that each of four amino acids (leucine 25, arginine 36, isoleucine 42, and glycine 59) contributes to DNA binding in the context of the N-terminal 123 amino acids of SB transposase, as indicated by electrophoretic mobility shift analysis, and to functional activity of the full-length transposase, as determined by a quantitative HeLa cell-based transposition assay. Moreover, we show that amino acid substitutions within either the putative oligomerization domain (L11A, L18A, L25A, and L32A) or the nuclear localization signal (K104A and R105A) severely impair its ability to mediate DNA transposition in mammalian cells. In contrast, each of 10 single amino acid changes within the bipartite DNA-binding domain is shown to greatly enhance SB's transpositional activity in mammalian cells. These hyperactive mutations functioned synergistically when combined and are shown to significantly improve transposase affinity for transposon end sequences. Finally, we show that enhanced DNA-binding activity results in improved cleavage kinetics, increased SB element mobilization from host cell chromosomes, and dramatically improved gene transfer capabilities of SB in vivo in mice. These studies provide important insights into vertebrate transposon biology and indicate that Sleeping Beauty can be readily improved for enhanced genetic research applications in mammals.
Figures
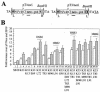
FIG. 1.
Effects of amino acid substitutions on the efficiency of SB transposition in human cells. (A) Schematic diagram of the SB transposase. Shown are the two parts of the pairlike DNA-binding domain (PAI and RED), the GRRR AT hook motif, the bipartite nuclear localization signal, and the catalytic core containing the conserved DD(35)E motif. A leucine zipper (L-L-L-L-V) shown to be involved in subunit multimerization overlaps the PAI domain. (B) Immunoblot analysis of SB10 and representative mutant transposase proteins expressed by plasmid transfection of HeLa cells. Protein extracts were prepared from cells 40 h posttransfection and subjected to electrophoresis and electroblotting, and the 40-kDa transposase was detected with a polyclonal rabbit antibody to the SB protein. (C) Relative transposition activity of SB10 and mutated SB transposases in HeLa cells. HeLa cells were cotransfected with a plasmid encoding a neomycin-marked transposon (pT/nori) together with a plasmid encoding no transposase (−), the standard SB10 transposase (SB), or a transposase missense mutant. Shown are the transpositional efficiencies of 95 alanine-scan transposase mutants relative to that of SB10, which was adjusted to 100%. The best hyperactive mutants identified after three independent experiments are shown as black columns. Numbers indicate residue positions, asterisks demark the leucine zipper motif, and boxes enclose the AT hook motif and the first two residues of the NLS.
FIG. 2.
Effects of single amino acid transposase mutations on transposon DNA-binding activities. (A) Alignment of SB's DR sequences with identical nucleotides shaded in gray. (B) The N123 peptides from SB10 and mutant transposases were complexed with double-stranded radiolabeled oligonucleotides encoding the outer DR (left column) or inner DR (right column) sequences. The numbers represent the amino acid residues in SB10 that were mutated to alanine. Abbreviations: −, no transposase control; SB+, SB10-DNA complexes formed in the presence of 500-fold unlabeled oligonucleotide as a specific competitor.
FIG. 3.
Effects on the frequency of SB element transposition when using hyperactive mutations in combination and in the presence of a hyperactive transposon. (A) Schematic overview of the first-generation (pT/nori) and hyperactive (pT3/nori) neomycin-marked transposon vectors. The pT3-derived vector contains an extra transposition enhancer domain via duplication of the left IR-DR structure and has an extra TA dinucleotide flanking the 3′ end of the element (underlined) to promote increased excision. (B) Transposition frequencies in HeLa cells using traditional SB10/pT and hyperactive transposase and transposon components. The effects of hyperactive mutations in combination are shown relative to those of the SB10 transposase. Gene transfer frequencies for each transposase used in combination with either pT/nori (white bars) or pT3/nori (shaded bars) are shown.
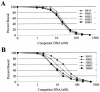
FIG. 4.
Comparison of the relative binding affinities of SB10 and HSB mutants for transposon binding sites. SB-N123 peptides for SB10 and hyperactive transposases were complexed with double-stranded radiolabeled oligonucleotides encoding the inner (A) or outer (B) DR sequences. Protein-DNA complexes were formed in the presence of increasing amounts of the corresponding unlabeled oligonucleotide. The binding percentage was determined using a PhosphorImager and normalized according to the amount of probe bound in the absence of any competitor minus the amount of probe bound at a 500 nM concentration.
FIG. 5.
Effects of amino acid substitutions on donor DNA cleavage activity in human cells. A transposon donor plasmid was transiently transfected into HeLa cells either alone or in combination with a transposase plasmid, and the DNA was isolated 30 h later for PCR analyses with primers flanking the donor element. Donor plasmids that have undergone _SB_-mediated transposon excision and double-strand break support the amplification of a 271-bp product. The amount of SB element excision produced in the presence of inactive (lanes 3-5) and hyperactive (lanes 6 to 8) SB mutants is shown relative to that produced in the presence of SB10.
FIG. 6.
Effect of transposase hyperactivity on chromosomal transposition rates. (A) Genetic assay for detecting rare chromosomal transposition events. A neomycin resistance gene (neo) under the control of simian virus 40 (SV40) promoter is inactivated by the insertion of a nonautonomous SB element containing a hygromycin resistance (hygR) gene driven by the thymidine kinase (TK) promoter. This construct is packaged into a lentivirus and randomly integrated as a single-copy provirus into the genomes of infected HeLa cells. Transient expression of active SB transposase in these cells results in excision of the SB element and activated expression of the neo gene, resulting in G418 drug-resistant growth. LTR, human immunodeficiency virus type 1 long terminal repeat; chrm, chromosome. (B) Transposition frequencies in HeLa cells as determined using SB10 and hyperactive transposase. Three different reporter cell lines were each transiently transfected with plasmids encoding green fluorescent protein (GFP) as a control, SB10, or HSB3. Cells were growth selected in G418 for 3 weeks, and the resulting colonies were fixed, stained, and counted. ND, none detected.
FIG. 7.
Comparison of transposition activities in mouse liver following administration of standard pT/SB10 and hyperactive SB systems. C57BL/6-scid mice (four to five per group) were injected via the tail vein with 1 μg of plasmids encoding no transposase, SB10, or an improved transposase (HSB2) together with 25 μg of either pT/βgeo (encoding a β-galactosidase-marked transposon) or the improved pT3/βgeo version. Mice were sacrificed 6 weeks later, and their livers were sectioned and stained for β-galactosidase expression to determine the mean number of X-Gal (5-bromo-4-chloro-3-indolyl-β-
d
-galactopyranoside)-positive hepatocytes observed under each experimental condition (shown ± standard deviations). The transposition efficiency for SB10 transposase plus pT/βgeo was adjusted to 100%, and other combinations are shown as relative activities. Noted above each bar are the percentages of transfected mouse hepatocytes that remained X-Gal positive 6 weeks later.
Similar articles
- Targeted Sleeping Beauty transposition in human cells.
Ivics Z, Katzer A, Stüwe EE, Fiedler D, Knespel S, Izsvák Z. Ivics Z, et al. Mol Ther. 2007 Jun;15(6):1137-44. doi: 10.1038/sj.mt.6300169. Epub 2007 Apr 10. Mol Ther. 2007. PMID: 17426709 - Counterselection and co-delivery of transposon and transposase functions for Sleeping Beauty-mediated transposition in cultured mammalian cells.
Converse AD, Belur LR, Gori JL, Liu G, Amaya F, Aguilar-Cordova E, Hackett PB, McIvor RS. Converse AD, et al. Biosci Rep. 2004 Dec;24(6):577-94. doi: 10.1007/s10540-005-2793-9. Biosci Rep. 2004. PMID: 16158196 - Development of hyperactive sleeping beauty transposon vectors by mutational analysis.
Zayed H, Izsvák Z, Walisko O, Ivics Z. Zayed H, et al. Mol Ther. 2004 Feb;9(2):292-304. doi: 10.1016/j.ymthe.2003.11.024. Mol Ther. 2004. PMID: 14759813 - Sleeping Beauty Transposition.
Ivics Z, Izsvák Z. Ivics Z, et al. Microbiol Spectr. 2015 Apr;3(2):MDNA3-0042-2014. doi: 10.1128/microbiolspec.MDNA3-0042-2014. Microbiol Spectr. 2015. PMID: 26104705 Review. - The Sleeping Beauty transposon toolbox.
Ammar I, Izsvák Z, Ivics Z. Ammar I, et al. Methods Mol Biol. 2012;859:229-40. doi: 10.1007/978-1-61779-603-6_13. Methods Mol Biol. 2012. PMID: 22367875 Review.
Cited by
- Assessing and engineering the IscB-ωRNA system for programmed genome editing.
Yan H, Tan X, Zou S, Sun Y, Ke A, Tang W. Yan H, et al. Nat Chem Biol. 2024 Jul 8. doi: 10.1038/s41589-024-01669-3. Online ahead of print. Nat Chem Biol. 2024. PMID: 38977787 - Oncogene-Driven Induction of Orthotopic Cholangiocarcinoma in Mice.
Plantureux C, Paillet J, Autret G, Pérez-Lanzón M, Kroemer G, Maiuri MC, Pol J. Plantureux C, et al. Methods Mol Biol. 2024;2769:99-108. doi: 10.1007/978-1-0716-3694-7_8. Methods Mol Biol. 2024. PMID: 38315392 - Mage transposon: a novel gene delivery system for mammalian cells.
Tian J, Tong D, Li Z, Wang E, Yu Y, Lv H, Hu Z, Sun F, Wang G, He M, Xia T. Tian J, et al. Nucleic Acids Res. 2024 Mar 21;52(5):2724-2739. doi: 10.1093/nar/gkae048. Nucleic Acids Res. 2024. PMID: 38300794 Free PMC article. - EGFR core fucosylation, induced by hepatitis C virus, promotes TRIM40-mediated-RIG-I ubiquitination and suppresses interferon-I antiviral defenses.
Pan Q, Xie Y, Zhang Y, Guo X, Wang J, Liu M, Zhang XL. Pan Q, et al. Nat Commun. 2024 Jan 22;15(1):652. doi: 10.1038/s41467-024-44960-6. Nat Commun. 2024. PMID: 38253527 Free PMC article. - Current strategies employed in the manipulation of gene expression for clinical purposes.
Tsai HC, Pietrobon V, Peng M, Wang S, Zhao L, Marincola FM, Cai Q. Tsai HC, et al. J Transl Med. 2022 Nov 18;20(1):535. doi: 10.1186/s12967-022-03747-3. J Transl Med. 2022. PMID: 36401279 Free PMC article. Review.
References
- Cui, Z., A. M. Geurts, G. Liu, C. D. Kaufman, and P. B. Hackett. 2002. Structure-function analysis of the inverted terminal repeats of the sleeping beauty transposon. J. Mol. Biol. 318:1221-1235. - PubMed
- Drabek, D., L. Zagoraiou, T. deWit, A. Langeveld, C. Roumpaki, C. Mamalaki, C. Savakis, and F. Grosveld. 2003. Transposition of the Drosophila hydei Minos transposon in the mouse germ line. Genomics 81:108-111. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources