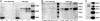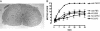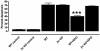Erythropoietin mediates tissue protection through an erythropoietin and common beta-subunit heteroreceptor - PubMed (original) (raw)
. 2004 Oct 12;101(41):14907-12.
doi: 10.1073/pnas.0406491101. Epub 2004 Sep 29.
Giovanni Grasso, Fabio Fiordaliso, Alessandra Sfacteria, Pietro Ghezzi, Maddalena Fratelli, Roberto Latini, Qiao-Wen Xie, John Smart, Chiao-Ju Su-Rick, Eileen Pobre, Deborah Diaz, Daniel Gomez, Carla Hand, Thomas Coleman, Anthony Cerami
Affiliations
- PMID: 15456912
- PMCID: PMC522054
- DOI: 10.1073/pnas.0406491101
Erythropoietin mediates tissue protection through an erythropoietin and common beta-subunit heteroreceptor
Michael Brines et al. Proc Natl Acad Sci U S A. 2004.
Abstract
The cytokine erythropoietin (Epo) is tissue-protective in preclinical models of ischemic, traumatic, toxic, and inflammatory injuries. We have recently characterized Epo derivatives that do not bind to the Epo receptor (EpoR) yet are tissue-protective. For example, carbamylated Epo (CEpo) does not stimulate erythropoiesis, yet it prevents tissue injury in a wide variety of in vivo and in vitro models. These observations suggest that another receptor is responsible for the tissue-protective actions of Epo. Notably, prior investigation suggests that EpoR physically interacts with the common beta receptor (betacR), the signal-transducing subunit shared by the granulocyte-macrophage colony stimulating factor, and the IL-3 and IL-5 receptors. However, because betacR knockout mice exhibit normal erythrocyte maturation, betacR is not required for erythropoiesis. We hypothesized that betacR in combination with the EpoR expressed by nonhematopoietic cells constitutes a tissue-protective receptor. In support of this hypothesis, membrane proteins prepared from rat brain, heart, liver, or kidney were greatly enriched in EpoR after passage over either Epo or CEpo columns but covalently bound in a complex with betacR. Further, antibodies against EpoR coimmunoprecipitated betacR from membranes prepared from neuronal-like P-19 cells that respond to Epo-induced tissue protection. Immunocytochemical studies of spinal cord neurons and cardiomyocytes protected by Epo demonstrated cellular colocalization of Epo betacR and EpoR. Finally, as predicted by the hypothesis, neither Epo nor CEpo was active in cardiomyocyte or spinal cord injury models performed in the betacR knockout mouse. These data support the concept that EpoR and betacR comprise a tissue-protective heteroreceptor.
Figures
Fig. 1.
Despite having no affinity for CEpo, EpoR is bound to a CEpo affinity column but within a complex. (A) Rat brain membrane proteins sequentially purified over a lentil lectin column and Epo or CEpo affinity columns were subjected to EpoR Western blotting in either a reduced or nonreduced state. Bands (≈64 kDa) consistent with the EpoR were visualized only under reduced conditions. S, soluble EpoR-positive control (29 kDa); E, Epo affinity column; C, CEpo affinity column. (B) Membranes prepared from heart, kidney (kid), and liver show results similar to those for brain membranes but as a distinct doublet. (C) In contrast, cell membranes obtained from TF-1 cells and run under nonreducing conditions show the doublet EpoR.
Fig. 2.
EpoR and βcR are present as a complex in neuronal lysates. Immunoprecipitation of membranes prepared from P19 mouse neuroblastoma cells demonstrate that either anti-EpoR or anti-βcR pulls down a protein consistent with βcR (≈130 kDa), as well as a smaller molecular species. Equivalent results were obtained in the presence or absence of Epo.
Fig. 3.
Cells exhibiting tissue protection express both the EpoR and βcR subunit. (A) Spinal cord neurons within the central gray obtained from normal mice show prominent staining of the somata for both proteins. (B) Choroid plexus (arrow), as well as the ependymal cell layer (arrowhead), exhibit prominent staining for both EpoR and βcR. EpoR staining appears punctate. Radial glia (double arrow) are also doubly immunoreactive. (C) Purkinje cells of the cerebellum stain densely for EpoR in a punctate manner (Left), whereas anti-βcR stains somata, as well as the proximal dendrites (arrow), more diffusely. Note that neuronal somata in the molecular layer, as well as granule cells, are also immunopositive for both proteins. Right illustrates negative control (primary antibody omitted). (D) Sections of myocardium obtained from normal mice show prominent EpoR and βcR immunostaining. Sections obtained from the βcR knockout mouse exhibit prominent EpoR immunoreactivity. CTL EpoR is a negative control in which the primary antibody was omitted.
Fig. 4.
CEpo restores motor function after spinal compression in wild-type mice but not in strain-matched βcR knockout mice. (A) Spinal cord morphology is normal in the βcR knockout mouse (hematoxylin/eosin-stained). (B) βcR knockout mice subjected to spinal cord compressive injury do not respond to either Epo or CEpo (10 μg/kg of body weight) given i.p. as a single dose immediately after injury.
Fig. 5.
rhEpo is tissue-protective of staurosporine-induced apoptosis for cardiomyocytes derived from wild-type cells but not identically prepared cells from βcR knockout mice. rhEpo was added (100 ng/ml) 30 min before the addition of staurosporine (0.1 μg/ml). Each condition corresponds to between four and eight replications. ***, P < 0.001 vs. staurosporine alone.
Similar articles
- Skin regeneration with conical and hair follicle structure of deep second-degree scalding injuries via combined expression of the EPO receptor and beta common receptor by local subcutaneous injection of nanosized rhEPO.
Bader A, Ebert S, Giri S, Kremer M, Liu S, Nerlich A, Günter CI, Smith DU, Machens HG. Bader A, et al. Int J Nanomedicine. 2012;7:1227-37. doi: 10.2147/IJN.S28186. Epub 2012 Mar 6. Int J Nanomedicine. 2012. PMID: 22419870 Free PMC article. - β Common receptor integrates the erythropoietin signaling in activation of endothelial nitric oxide synthase.
Su KH, Shyue SK, Kou YR, Ching LC, Chiang AN, Yu YB, Chen CY, Pan CC, Lee TS. Su KH, et al. J Cell Physiol. 2011 Dec;226(12):3330-9. doi: 10.1002/jcp.22678. J Cell Physiol. 2011. PMID: 21321940 - Signaling pathways of cell proliferation are involved in the differential effect of erythropoietin and its carbamylated derivative.
Chamorro ME, Wenker SD, Vota DM, Vittori DC, Nesse AB. Chamorro ME, et al. Biochim Biophys Acta. 2013 Aug;1833(8):1960-8. doi: 10.1016/j.bbamcr.2013.04.006. Epub 2013 Apr 16. Biochim Biophys Acta. 2013. PMID: 23602701 - Erythropoietin Receptor/β Common Receptor: A Shining Light on Acute Kidney Injury Induced by Ischemia-Reperfusion.
Wu Y, Yang B. Wu Y, et al. Front Immunol. 2021 Jun 30;12:697796. doi: 10.3389/fimmu.2021.697796. eCollection 2021. Front Immunol. 2021. PMID: 34276689 Free PMC article. Review. - Alternative Erythropoietin Receptors in the Nervous System.
Ostrowski D, Heinrich R. Ostrowski D, et al. J Clin Med. 2018 Feb 2;7(2):24. doi: 10.3390/jcm7020024. J Clin Med. 2018. PMID: 29393890 Free PMC article. Review.
Cited by
- Erythropoietin attenuates cardiac dysfunction in experimental sepsis in mice via activation of the β-common receptor.
Khan AI, Coldewey SM, Patel NS, Rogazzo M, Collino M, Yaqoob MM, Radermacher P, Kapoor A, Thiemermann C. Khan AI, et al. Dis Model Mech. 2013 Jul;6(4):1021-30. doi: 10.1242/dmm.011908. Epub 2013 Mar 15. Dis Model Mech. 2013. PMID: 23519033 Free PMC article. - Erythropoietin in Lupus: Unanticipated Immune Modulating Effects of a Kidney Hormone.
Eswarappa M, Cantarelli C, Cravedi P. Eswarappa M, et al. Front Immunol. 2021 Mar 16;12:639370. doi: 10.3389/fimmu.2021.639370. eCollection 2021. Front Immunol. 2021. PMID: 33796104 Free PMC article. Review. - Delayed administration of pyroglutamate helix B surface peptide (pHBSP), a novel nonerythropoietic analog of erythropoietin, attenuates acute kidney injury.
Patel NS, Kerr-Peterson HL, Brines M, Collino M, Rogazzo M, Fantozzi R, Wood EG, Johnson FL, Yaqoob MM, Cerami A, Thiemermann C. Patel NS, et al. Mol Med. 2012 May 9;18(1):719-27. doi: 10.2119/molmed.2012.00093. Mol Med. 2012. PMID: 22415011 Free PMC article. - Attenuation of inflammation and apoptosis by pre- and posttreatment of darbepoetin-alpha in acute liver failure of mice.
Le Minh K, Klemm K, Abshagen K, Eipel C, Menger MD, Vollmar B. Le Minh K, et al. Am J Pathol. 2007 Jun;170(6):1954-63. doi: 10.2353/ajpath.2007.061056. Am J Pathol. 2007. PMID: 17525263 Free PMC article. - Differential modulation of angiogenesis by erythropoiesis-stimulating agents in a mouse model of ischaemic retinopathy.
McVicar CM, Colhoun LM, Abrahams JL, Kitson CL, Hamilton R, Medina RJ, Durga D, Gardiner TA, Rudd PM, Stitt AW. McVicar CM, et al. PLoS One. 2010 Jul 29;5(7):e11870. doi: 10.1371/journal.pone.0011870. PLoS One. 2010. PMID: 20686695 Free PMC article.
References
- Grasso, G., Sfacteria, A., Cerami, A. & Brines, M. (2004) Neuroscientist 10, 93-98. - PubMed
- Ghezzi, P. & Brines, M. (2004) Cell Death Differ. 11, Suppl. 1, S37-S44. - PubMed
- Livnah, O., Stura, E. A., Middleton, S. A., Johnson, D. L., Jolliffe, L. K. & Wilson, I. A. (1999) Science 283, 987-990. - PubMed
- Masuda, S., Nagao, M., Takahata, K., Konishi, Y., Gallyas, F., Jr., Tabira, T. & Sasaki, R. (1993) J. Biol. Chem. 268, 11208-11216. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials