The GABAA receptor gamma2 subunit R43Q mutation linked to childhood absence epilepsy and febrile seizures causes retention of alpha1beta2gamma2S receptors in the endoplasmic reticulum - PubMed (original) (raw)
The GABAA receptor gamma2 subunit R43Q mutation linked to childhood absence epilepsy and febrile seizures causes retention of alpha1beta2gamma2S receptors in the endoplasmic reticulum
Jing-Qiong Kang et al. J Neurosci. 2004.
Erratum in
- J Neurosci. 2004 Oct 13;24(41):1p following 9126. Kang, Jingqiong [corrected to Kang, Jing-Qiong]
Abstract
The GABA(A) receptor gamma2 subunit mutation R43Q is an autosomal dominant mutation associated with childhood absence epilepsy and febrile seizures. Previously, we demonstrated that homozygous alpha1beta3gamma2L(R43Q) receptor whole-cell currents had reduced amplitude with unaltered time course, suggesting reduced cell surface expression of functional receptors. In human embryonic kidney 293-T cells, we demonstrate that both heterozygous and homozygous alpha1beta2gamma2S(R43Q) GABA(A) receptor current amplitudes were reduced when receptors were assembled from coexpressed alpha1, beta2, and gamma2S subunits and from beta2-alpha1 tandem subunits coexpressed with the gamma2L subunit. Using fluorescence confocal microscopy, we demonstrated that mutant receptors containing enhanced yellow fluorescent protein-tagged gamma2S subunits had reduced surface expression and were retained in the endoplasmic reticulum. In addition, using biotinylation of surface receptors and immunoblotting, we confirmed that alpha1beta2gamma2S(R43Q) receptors had reduced surface expression. These results provide evidence that the gamma2S(R43Q) mutation impaired GABA(A) receptor function by compromising receptor trafficking and reducing surface expression.
Figures
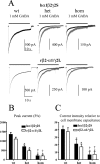
Figure 1.
Heterozygous and homozygous α1β2γ2S(R43Q) receptors had unaltered diazepam sensitivity. A, Whole-cell recordings were made from lifted HEK293-T cells expressing wild-type (wt) hα1β2γ2S or heterozygous (het) or homozygous (hom) hα1β2γ2S(R43Q) receptors. An approximate EC20 value of GABA concentration (2 μ
m
) was applied for 6 sec to cells voltage clamped at -50 mV, and 1 μ
m
diazepam was coapplied with GABA. B, Diazepam enhancement is shown as a percentage of control current (average peak current before and after diazepam coapplication). The γ2S(R43Q) mutation did not alter the magnitude of diazepam enhancement for either heterozygous or homozygous expression with both free hα1β2γ2S assembly and forced rβ2-α1/γ2L assembly (n = 6 for each group).
Figure 2.
Heterozygous and homozygous α1β2γ2S(R43Q) receptors had reduced current amplitudes. A, Whole-cell currents were obtained from either hα1β2γ2S (wt), hα1β2γ2S(R43Q) (het), or hα1β2γ2S (R43Q) (hom) receptors in free assembly or forced rβ2-α1γ2L assembly. GABA (1 m
m
) was applied for 28 sec. Currents are shown to scale (dark traces) and normalized to wild-type currents (gray traces). The time scale for the first trace applies to all traces. B, C, Peak amplitudes (B) and relative current intensities (C) of heterozygous and homozygous hα1β2γ2S(R43Q) and rβ2-α1γ2L(R43Q) receptor currents were significantly reduced with both free and forced assembly (*p < 0.01 vs wild type; †p < 0.01 vs heterozygous; data are from 9-17 patches from 4 batches of cells).
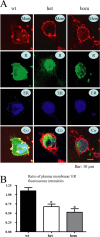
Figure 3.
Heterozygous and homozygous hα1β2γ2S(R43Q) receptors were trapped in the ER. A, Representative confocal fluorescence images of Cos-7 cells transfected with hα1β2γ2S-EYFP or hα1β2γ2S(R43Q)-EYFP receptors are presented. R, Receptor; Co, colocalized image. Wt α1β2γ2S-EYFP receptors were primarily in the cell membrane. Heterozygous (het) α1β2γ2S(R43Q)-EYFP receptors were found in both membrane and intracellular compartments. Homozygous (hom)α1β2γ2S(R43Q)-EYFP receptors were found primarily in intracellular compartments with minimal cell surface localization. Both heterozygous and homozygous receptors had a fluorescence pattern that was similar in distribution to the pECFP-ER fluorescence pattern (
supplemental material
, available at
). In B, the relative membrane/ER fluorescence intensity ratios for heterozygous and homozygous receptors were significantly reduced compared with that for the wild-type receptors (see Materials and Methods) (for each group, 9-11 randomly chosen cells were measured from 5 batches; *p < 0.01 vs wild type).
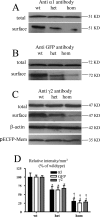
Figure 4.
Heterozygous and homozygous hα1β2γ2S(R43Q) receptors had reduced surface expression on HEK293-T cells. A, B, HEK293-T cells transfected with wild-type (wt) or heterozygous (het) or homozygous (hom) hα1β2γ2S(R43Q) receptors were biotinylated and immunoblotted with antibodies against the α1 subunit (A) and GFP variant EYFP (B). Expression of heterozygous or homozygous α1β2γ2S(R43Q) receptors resulted in similar levels of whole-cell protein expression (total) but reduced cell surface protein expression (surface) compared with wild-type receptors. C, HEK293-T cells transfected with wild-type or heterozygous or homozygous α1β2S(R43Q) receptors and pECFP-Mem were biotinylated, and whole-cell protein was precipitated with antibody against the human α1 subunit and detected by antibody against the γ2 subunit. Heterozygous and homozygous α1β2γ2S(R43Q) receptors revealed a similar protein expression in whole-cell level (total) but a reduced protein expression in the cell surface (surface). β-Actin was used as a control to demonstrate that equal amounts of protein were loaded (β-actin). Similarly, pECFP-Mem protein expression was also similar in each group (pECFP-Mem). D, The optical absorbency of Western blots was quantified with Bio-Rad QuantifyOne. In each group, heterozygous protein intensities were lower than wild type but higher than homozygous receptors (*p < 0.05 vs wild type; †p < 0.05 vs heterozygous; data are from 5 experiments).
Comment in
- GABA receptors gone bad: the wrong place at the wrong time.
Lagrange A. Lagrange A. Epilepsy Curr. 2005 May-Jun;5(3):91-4. doi: 10.1111/j.1535-7511.2005.05304.x. Epilepsy Curr. 2005. PMID: 16145612 Free PMC article. No abstract available.
Similar articles
- A gamma 2(R43Q) mutation, linked to epilepsy in humans, alters GABAA receptor assembly and modifies subunit composition on the cell surface.
Frugier G, Coussen F, Giraud MF, Odessa MF, Emerit MB, Boué-Grabot E, Garret M. Frugier G, et al. J Biol Chem. 2007 Feb 9;282(6):3819-28. doi: 10.1074/jbc.M608910200. Epub 2006 Dec 5. J Biol Chem. 2007. PMID: 17148443 - The GABRG2 mutation, Q351X, associated with generalized epilepsy with febrile seizures plus, has both loss of function and dominant-negative suppression.
Kang JQ, Shen W, Macdonald RL. Kang JQ, et al. J Neurosci. 2009 Mar 4;29(9):2845-56. doi: 10.1523/JNEUROSCI.4772-08.2009. J Neurosci. 2009. PMID: 19261880 Free PMC article. - Molecular Pathogenic Basis for GABRG2 Mutations Associated With a Spectrum of Epilepsy Syndromes, From Generalized Absence Epilepsy to Dravet Syndrome.
Kang JQ, Macdonald RL. Kang JQ, et al. JAMA Neurol. 2016 Aug 1;73(8):1009-16. doi: 10.1001/jamaneurol.2016.0449. JAMA Neurol. 2016. PMID: 27367160 Free PMC article. Review. - Mutant GABA(A) receptor subunits in genetic (idiopathic) epilepsy.
Hirose S. Hirose S. Prog Brain Res. 2014;213:55-85. doi: 10.1016/B978-0-444-63326-2.00003-X. Prog Brain Res. 2014. PMID: 25194483 Review.
Cited by
- Endogenous positive allosteric modulation of GABA(A) receptors by diazepam binding inhibitor.
Christian CA, Herbert AG, Holt RL, Peng K, Sherwood KD, Pangratz-Fuehrer S, Rudolph U, Huguenard JR. Christian CA, et al. Neuron. 2013 Jun 19;78(6):1063-74. doi: 10.1016/j.neuron.2013.04.026. Epub 2013 May 30. Neuron. 2013. PMID: 23727119 Free PMC article. - A missense mutation in SLC6A1 associated with Lennox-Gastaut syndrome impairs GABA transporter 1 protein trafficking and function.
Cai K, Wang J, Eissman J, Wang J, Nwosu G, Shen W, Liang HC, Li XJ, Zhu HX, Yi YH, Song J, Xu D, Delpire E, Liao WP, Shi YW, Kang JQ. Cai K, et al. Exp Neurol. 2019 Oct;320:112973. doi: 10.1016/j.expneurol.2019.112973. Epub 2019 Jun 6. Exp Neurol. 2019. PMID: 31176687 Free PMC article. - The GABRA6 mutation, R46W, associated with childhood absence epilepsy, alters 6β22 and 6β2 GABA(A) receptor channel gating and expression.
Hernandez CC, Gurba KN, Hu N, Macdonald RL. Hernandez CC, et al. J Physiol. 2011 Dec 1;589(Pt 23):5857-78. doi: 10.1113/jphysiol.2011.218883. Epub 2011 Sep 19. J Physiol. 2011. PMID: 21930603 Free PMC article. - Single-cell genetic expression of mutant GABAA receptors causing Human genetic epilepsy alters dendritic spine and GABAergic bouton formation in a mutation-specific manner.
Lachance-Touchette P, Choudhury M, Stoica A, Di Cristo G, Cossette P. Lachance-Touchette P, et al. Front Cell Neurosci. 2014 Oct 14;8:317. doi: 10.3389/fncel.2014.00317. eCollection 2014. Front Cell Neurosci. 2014. PMID: 25352779 Free PMC article. - SYVN1, an ERAD E3 Ubiquitin Ligase, Is Involved in GABAAα1 Degradation Associated with Methamphetamine-Induced Conditioned Place Preference.
Jiao DL, Chen Y, Liu Y, Ju YY, Long JD, Du J, Yu CX, Wang YJ, Zhao M, Liu JG. Jiao DL, et al. Front Mol Neurosci. 2017 Oct 5;10:313. doi: 10.3389/fnmol.2017.00313. eCollection 2017. Front Mol Neurosci. 2017. PMID: 29051727 Free PMC article.
References
- Baumann SW, Baur R, Sigel E (2001) Subunit arrangement of γ-aminobutyric acid type A receptors. J Biol Chem 276: 36275-36280. - PubMed
- Boileau AJ, Li T, Benkwitz C, Czajkowski C, Pearce RA (2003) Effects of γ2S subunit incorporation on GABAA receptor macroscopic kinetics. Neuropharmacology 44: 1003-1012. - PubMed
- Bolte S, Talbot C, Boutte Y, Catrice O, Read ND, Satiat-Jeunemaitre B (2004) FM-dyes as experimental probes for dissecting vesicle trafficking in living plant cells. J Microsc 214: 159-173. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources