Accelerating drug discovery - PubMed (original) (raw)
Accelerating drug discovery
Sandra Kraljevic et al. EMBO Rep. 2004 Sep.
Abstract
Although the evolution of '-omics' methodologies is still in its infancy, both the pharmaceutical industry and patients could benefit from their implementation in the drug development process
Figures
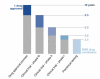
Figure 1
Current time-scale of drug approval process. New drugs are developed through several phases: synthesis and extraction of new compounds, biological screening and pharmacological testing, pharmaceutical dosage formulation and stability testing, toxicology and safety testing, phase I, II and III clinical evaluation process, development for manufacturing and quality control, bioavailability studies and post-approval research. Before testing in humans can start, a significant body of pre-clinical data must be compiled, and appropriate toxic doses should be found for further in vivo testing to ensure human safety. Toxicology, pharmacology, metabolism and pharmaceutical sciences represent the core of pre-clinical development.
Figure 2
The increasing availability of quantitative biological data from the human genome project, coupled with advances in instrumentation, reagents, methodologies, bioinformatics tools and software, are transforming the ways drug discovery and drug development are performed. The ability to combine high-throughput genomic, proteomic, metabolomic and other experimental approaches with drug discovery will speed up the development of safer, more effective and better-targeted therapeutic agents. Functional genomics approaches should be exploited throughout the entire drug development process. Particularly, combinatorial chemistry, in silico structure prediction, new scaffold-like molecular weight compounds targeting conserved regions of multiple protein family members, accompanied by high-throughput X-ray crystallography and proteomic-based drug target discovery, will reduce the time required for drug discovery. Large-scale (robotics) in vitro screening using cultured human cell lines and in vivo studies on 'humanized' mouse models combined with functional genomic analysis of different organs will speed up testing. Finally, pharmacogenomics-guided clinical trials, followed by toxicogenomics-based analyses should shorten the clinical phase of testing by as much as 3–4 years.
Figure 3
New chemical approaches and biological assays combined with bioinformatics provide a general ability to globally assess many classes of cellular and other molecules. Such attempts are likely to expand the repertoire of potential therapeutics directed towards a particular molecular target in the near future.
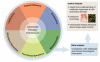
Figure 4
The advantages of '-omics' approaches in the drug development process
Similar articles
- Deconstructing the drug development process: the new face of innovation.
Kaitin KI. Kaitin KI. Clin Pharmacol Ther. 2010 Mar;87(3):356-61. doi: 10.1038/clpt.2009.293. Epub 2010 Feb 3. Clin Pharmacol Ther. 2010. PMID: 20130565 Free PMC article. Review. - A comparison of FDA and EMA drug approval: implications for drug development and cost of care.
Howie LJ, Hirsch BR, Abernethy AP. Howie LJ, et al. Oncology (Williston Park). 2013 Dec;27(12):1195, 1198-1200, 1202 passim. Oncology (Williston Park). 2013. PMID: 24624536 No abstract available. - Eyes on New Product Development.
Novack GD. Novack GD. J Ocul Pharmacol Ther. 2017 Mar;33(2):65. doi: 10.1089/jop.2017.29024.gdn. Epub 2017 Jan 18. J Ocul Pharmacol Ther. 2017. PMID: 28099041 Review. No abstract available. - Effective global drug development strategy for obtaining regulatory approval in Japan in the context of ethnicity-related drug response factors.
Ichimaru K, Toyoshima S, Uyama Y. Ichimaru K, et al. Clin Pharmacol Ther. 2010 Mar;87(3):362-6. doi: 10.1038/clpt.2009.285. Epub 2010 Jan 27. Clin Pharmacol Ther. 2010. PMID: 20107436 Review. No abstract available. - Pharmaceutical biotechnology products approved within the European Union.
Walsh G. Walsh G. Eur J Pharm Biopharm. 2003 Jan;55(1):3-10. doi: 10.1016/s0939-6411(02)00165-0. Eur J Pharm Biopharm. 2003. PMID: 12551698 Review.
Cited by
- Pharmacogenetics: An Important Part of Drug Development with A Focus on Its Application.
Oates JT, Lopez D. Oates JT, et al. Int J Biomed Investig. 2018;1(2):111. doi: 10.31531/2581-4745.1000111. Epub 2018 May 27. Int J Biomed Investig. 2018. PMID: 32467882 Free PMC article. - Multi-targeted therapy of cancer by niclosamide: A new application for an old drug.
Li Y, Li PK, Roberts MJ, Arend RC, Samant RS, Buchsbaum DJ. Li Y, et al. Cancer Lett. 2014 Jul 10;349(1):8-14. doi: 10.1016/j.canlet.2014.04.003. Epub 2014 Apr 13. Cancer Lett. 2014. PMID: 24732808 Free PMC article. Review. - From function to translation: Decoding genetic susceptibility to human diseases via artificial intelligence.
Long E, Wan P, Chen Q, Lu Z, Choi J. Long E, et al. Cell Genom. 2023 May 4;3(6):100320. doi: 10.1016/j.xgen.2023.100320. eCollection 2023 Jun 14. Cell Genom. 2023. PMID: 37388909 Free PMC article. Review. - Niclosamide inhibition of STAT3 synergizes with erlotinib in human colon cancer.
Shi L, Zheng H, Hu W, Zhou B, Dai X, Zhang Y, Liu Z, Wu X, Zhao C, Liang G. Shi L, et al. Onco Targets Ther. 2017 Mar 23;10:1767-1776. doi: 10.2147/OTT.S129449. eCollection 2017. Onco Targets Ther. 2017. PMID: 28367059 Free PMC article. - Artificial Intelligence and Quantum Computing as the Next Pharma Disruptors.
Cova T, Vitorino C, Ferreira M, Nunes S, Rondon-Villarreal P, Pais A. Cova T, et al. Methods Mol Biol. 2022;2390:321-347. doi: 10.1007/978-1-0716-1787-8_14. Methods Mol Biol. 2022. PMID: 34731476
References
- Aardema MJ, MacGregor JT (2002) Toxicology and genetic toxicology in the new era of “toxicogenomics”: impact of “-omics” technologies. Mutat Res 499: 13–25 - PubMed
- Ball CA et al. ; Microarray Gene Expression Data (MGED) Society (2002) Standards for microarray data. Science 298: 539. - PubMed
- Banks RE, Dunn MJ, Hochstrasser DF, Sanchez JC, Blackstock W, Pappin DJ, Selby PJ (2000) Proteomics: new perspectives, new biomedical opportunities. Lancet 356: 1749–1756 - PubMed
- Boorman GA, Anderson SP, Casey WM, Brown RH, Crosby LM, Gottschalk K, Easton M, Ni H, Morgan KT (2002) Toxicogenomics, drug discovery, and the pathologist. Toxicol Pathol 30: 15–27 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical