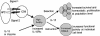IL-15/IL-15Ralpha-mediated avidity maturation of memory CD8+ T cells - PubMed (original) (raw)
IL-15/IL-15Ralpha-mediated avidity maturation of memory CD8+ T cells
SangKon Oh et al. Proc Natl Acad Sci U S A. 2004.
Abstract
T cell avidity is critical to viral clearance, but mechanisms of CD8(+) T cell avidity maturation are poorly understood. Here, we find that IL-15 mediates two mechanisms of avidity maturation. (i) By selection at the population level, IL-15 promotes greater survival of high- compared with low-avidity cytotoxic T lymphocytes (CTLs). High-avidity CTLs express higher levels of IL-15Ralpha and persist longer by homeostatic proliferation. (ii) At the individual cell level, IL-15 induces higher levels of surface coreceptor CD8alphabeta, increasing functional avidity. IL-15 during priming selects or induces higher-avidity CTLs. Conversely, high-avidity CTLs are diminished in IL-15Ralpha knockout mice. These results provide an explanation of CD8+ T cell avidity maturation and may contribute to the design of novel vaccines.
Figures
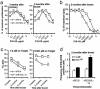
Fig. 1.
IL-15 during priming results in the long-lasting high-avidity CD8+ CTLs. (a) Spleen cells from three to four mice in each group were pooled, CD8+ T cells were positively purified and stimulated with splenocytes loaded with different concentrations of P18-I10, and proliferation was measured as described in Materials and Methods. Data are representative of three repeated experiments with consistent results. Each data point indicates mean ± SEM of triplicate assay. (b and c) Spleen CD8+ T cells from three to four mice in each group were pooled and stimulated with 1.0 (c Left) or 0.001 μM(b and c Right) P18-I10-pulsed splenocytes for 1 week. Lytic activity of CD8+ CTLs against P815 cells pulsed with different concentrations of the peptide was measured. Data represent mean ± SEM of triplicate assay at an effector-to-target cell ratio of 20:1. Similar kinetics of response were observed at all effector-to-target cell ratios tested, 80:1, 40:1, 20:1, and 10:1 (data not shown) in three repeat experiments. Data presented in b were normalized to the maximum lysis to compare avidity independent of magnitude of lysis and show mean ± SEM of triplicate assays. (d) Spleen CD8+ T cells from mice immunized as indicated were restimulated with 1.0 or 0.001 μM soluble P18-I10 overnight in the presence of brefeldin A. Cells were stained for intracellular IFN-γ by the manufacturer's protocol.
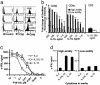
Fig. 2.
In vivo proliferation and persistence of antigen-specific CD8+ T cells depend on CD8+ T cell avidity. (a) Two to 3 months after boosting, splenocytes were stained with anti-CD8 and P18-I10 tetramer concurrently, as described in Materials and Methods. Cells at Left were from unimmunized animals. The three graphs at Right show the distribution of cells from gate R2, R3, and R4 in forward and side scatter plots. (b) Cells were gated, based on the brightness of tetramer staining, and sorted, left in the media for 6 h, and stimulated with P18-I10-pulsed splenocytes. Proliferation was measured as in Fig. 1. (c) Four months after boosting, spleen CD8+ T cells from mice immunized with vPE16 (Left) or vPE16/IL-15 (Right) were stained with anti-CD8 and P18-I10 tetramer, and then double-positive cells were sorted and reanalyzed by flow cytometry. (d) Two to 3 months after the boost, spleen CD8+ T cells from the immunized mice were labeled with CFSE and transferred to naïve animals. Four to 5 weeks after the transfer, spleen cells in the recipients were stained with anti-CD8 and tetramer, gated as in a, and analyzed to measure homeostatic proliferation. The numbers in histograms are mean ± SEM of at least four individual recipients in each experiment. Three repeated experiments showed consistent results.
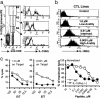
Fig. 4.
High-avidity CD8+ CTLs express higher levels of CD8αβ and IL-15 up-regulates the expression level of CD8αβ, resulting in enhanced functional activity. (a) Cells from the immunized mice were stained with anti-CD8 and P18-I10 tetramer, gated on the brightness of tetramer staining as in Fig. 2, and assessed for CD8β and TCRβ levels. (Top, Middle, and Bottom) High-, intermediate-, and low-avidity gates, respectively. Data are representative of three experiments with consistent results. (b) High- and low-avidity CD8+ CTL lines were incubated in media containing different concentrations of IL-15, and the expression levels of CD8β (Left) and CD8α (Center) were measured at 36 h of incubation. As a control, the levels of CD3 in both high- and low-avidity CTL lines were also measured (Right) when lines were incubated in the media containing 250 ng/ml IL-15. Data are mean ± SEM of three experiments. The fold increase of geometric mean fluorescence intensity (MFI) was calculated by (MFI with IL-15)/(MFI without IL-15). (c) The high-avidity CTL line, grown with 0.001 μM peptide, was stimulated with splenocytes pulsed with different concentrations of P18-I10 in the presence of IL-2 50 units/ml, IL-15 20 ng/ml, or both for 36 h, and the amount of IFN-γ produced was measured by ELISA. (d) The high- and low-avidity CD8+ T cell lines were depleted of presenting cells by positive selection with anti-CD8 beads and cultured without antigen or other cells in the presence of IL-2 50 units/ml, IL-15 20 ng/ml, or both for 36 h, and the amount of IFN-γ produced was measured by ELISA.
Fig. 3.
High-avidity CD8+ CTLs express higher levels of IL-15Rα, resulting in long-lasting memory CTLs. (a) Cells from the pooled spleens of three to four mice immunized with vPE16/IL-15 were stained with anti-CD8 and P18-I10 tetramer. IL-15Rα expression levels were measured for CD8+ T cells gated by the brightness of tetramer staining. Thin lines represent isotype control antibody. Three repeated experiments showed consistent data. (b) CD8+ T cell lines were raised by in vitro stimulation with different concentrations of P18-I10 in the media containing 50 units/ml IL-2. Cells grown in 0.0001, 0.01, and 1.0 μM peptide are designated as high-, intermediate-, and low-avidity CTL lines, respectively. The levels of IL-15Rα in CTL lines were measured 6-8 days after restimulation. Four repeated experiments showed consistent data. Data in Fig. 9 indicate that a high-avidity CTL line shows enhanced proliferative responsiveness to IL-15. (c) On day 17 after the immunization with vaccinia expressing ovalbumin, CD8+ T cells from the pooled spleens of three mice per group [B6 or IL-15Rα (-/-)] were restimulated with 1.0 or 0.001 μM of the dominant epitope SIINFEKL peptide for 1 week. (Left) Functional activity of CD8+ CTLs against target cells pulsed with 1.0 or 0.001 μM of the peptide was measured by 5h 51Cr-release assay. (Right) CD8+ T cells were stimulated with 0.01 μM peptide, and lytic activities against target cells pulsed with different concentrations of peptide were measured. Data presented at Right were normalized to the maximum lysis to compare avidity independent of magnitude of lysis and show mean ± SEM of triplicate assays. Data were consistent in two repeat experiments. E/T, effector-to-target cell ratio.
Fig. 5.
Proposed mechanism by which IL-15 mediates antigen-independent avidity maturation of CD8+ T cells by both instruction at the individual cell level and selection at the population level. High-avidity CTLs are induced to express high levels of IL-15Rα, either by a strong signal through the TCR and costimulatory receptors (signals 1 and 2, respectively) or by selection or induction by IL-15 during priming (IL-15 in a vaccine or produced by dendritic cells). These high-avidity CTLs are thus more sensitive to low endogenous levels of IL-15, which then promotes preferential survival and homeostatic proliferation of the high-avidity CTLs, resulting in selection of a higher average avidity at the population level, or induces up-regulation of CD8αβ coreceptor, resulting in increased functional avidity at the individual cell level.
Similar articles
- Immunisation route-dependent expression of IL-4/IL-13 can modulate HIV-specific CD8(+) CTL avidity.
Ranasinghe C, Ramshaw IA. Ranasinghe C, et al. Eur J Immunol. 2009 Jul;39(7):1819-30. doi: 10.1002/eji.200838995. Eur J Immunol. 2009. PMID: 19582753 - Transregulation of memory CD8 T-cell proliferation by IL-15Ralpha+ bone marrow-derived cells.
Schluns KS, Klonowski KD, Lefrançois L. Schluns KS, et al. Blood. 2004 Feb 1;103(3):988-94. doi: 10.1182/blood-2003-08-2814. Epub 2003 Sep 25. Blood. 2004. PMID: 14512307 - Coordinate expression and trans presentation of interleukin (IL)-15Ralpha and IL-15 supports natural killer cell and memory CD8+ T cell homeostasis.
Burkett PR, Koka R, Chien M, Chai S, Boone DL, Ma A. Burkett PR, et al. J Exp Med. 2004 Oct 4;200(7):825-34. doi: 10.1084/jem.20041389. Epub 2004 Sep 27. J Exp Med. 2004. PMID: 15452177 Free PMC article. - IL-15 and IL-15Ralpha in CD4+T cell immunity.
Van Belle T, Grooten J. Van Belle T, et al. Arch Immunol Ther Exp (Warsz). 2005 Mar-Apr;53(2):115-26. Arch Immunol Ther Exp (Warsz). 2005. PMID: 15928580 Review. - Homeostasis of memory T cells.
Surh CD, Boyman O, Purton JF, Sprent J. Surh CD, et al. Immunol Rev. 2006 Jun;211:154-63. doi: 10.1111/j.0105-2896.2006.00401.x. Immunol Rev. 2006. PMID: 16824125 Review.
Cited by
- Signalling mechanisms driving homeostatic and inflammatory effects of interleukin-15 on tissue lymphocytes.
Skariah N, James OJ, Swamy M. Skariah N, et al. Discov Immunol. 2024 Jan 30;3(1):kyae002. doi: 10.1093/discim/kyae002. eCollection 2024. Discov Immunol. 2024. PMID: 38405398 Free PMC article. Review. - Protective heterologous T cell immunity in COVID-19 induced by the trivalent MMR and Tdap vaccine antigens.
Mysore V, Cullere X, Settles ML, Ji X, Kattan MW, Desjardins M, Durbin-Johnson B, Gilboa T, Baden LR, Walt DR, Lichtman AH, Jehi L, Mayadas TN. Mysore V, et al. Med. 2021 Sep 10;2(9):1050-1071.e7. doi: 10.1016/j.medj.2021.08.004. Epub 2021 Aug 14. Med. 2021. PMID: 34414383 Free PMC article. - Constant regulation for stable CD8 T-cell functional avidity and its possible implications for cancer immunotherapy.
Gilfillan CB, Hebeisen M, Rufer N, Speiser DE. Gilfillan CB, et al. Eur J Immunol. 2021 Jun;51(6):1348-1360. doi: 10.1002/eji.202049016. Epub 2021 Mar 30. Eur J Immunol. 2021. PMID: 33704770 Free PMC article. Review. - TCR Dependent Metabolic Programming Regulates Autocrine IL-4 Production Resulting in Self-Tuning of the CD8+ T Cell Activation Setpoint.
Crofts KF, Holbrook BC, Soto-Pantoja DR, Ornelles DA, Alexander-Miller MA. Crofts KF, et al. Front Immunol. 2020 Mar 31;11:540. doi: 10.3389/fimmu.2020.00540. eCollection 2020. Front Immunol. 2020. PMID: 32300344 Free PMC article. - In vivo cancer vaccination: Which dendritic cells to target and how?
Chiang CL, Kandalaft LE. Chiang CL, et al. Cancer Treat Rev. 2018 Dec;71:88-101. doi: 10.1016/j.ctrv.2018.10.012. Epub 2018 Oct 25. Cancer Treat Rev. 2018. PMID: 30390423 Free PMC article. Review.
References
- Derby, M. A., Alexander-Miller, M. A., Tse, R. & Berzofsky, J. A. (2001) J. Immunol. 166, 1690-1697. - PubMed
- Yee, C., Savage, P. A., Lee, P. P., Davis, M. M. & Greenberg, P. D. (1999) J. Immunol. 162, 2227-2234. - PubMed
- Zeh, H. J., III, Perry-Lalley, D., Dudley, M. E., Rosenberg, S. A. & Yang, J. C. (1999) J. Immunol. 162, 989-994. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials