Yeast Miro GTPase, Gem1p, regulates mitochondrial morphology via a novel pathway - PubMed (original) (raw)
Yeast Miro GTPase, Gem1p, regulates mitochondrial morphology via a novel pathway
Rebecca L Frederick et al. J Cell Biol. 2004.
Abstract
Cell signaling events elicit changes in mitochondrial shape and activity. However, few mitochondrial proteins that interact with signaling pathways have been identified. Candidates include the conserved mitochondrial Rho (Miro) family of proteins, which contain two GTPase domains flanking a pair of calcium-binding EF-hand motifs. We show that Gem1p (yeast Miro; encoded by YAL048C) is a tail-anchored outer mitochondrial membrane protein. Cells lacking Gem1p contain collapsed, globular, or grape-like mitochondria. We demonstrate that Gem1p is not an essential component of characterized pathways that regulate mitochondrial dynamics. Genetic studies indicate both GTPase domains and EF-hand motifs, which are exposed to the cytoplasm, are required for Gem1p function. Although overexpression of a mutant human Miro protein caused increased apoptotic activity in cultured cells (Fransson et al., 2003. J. Biol. Chem. 278:6495-6502), Gem1p is not required for pheromone-induced yeast cell death. Thus, Gem1p defines a novel mitochondrial morphology pathway which may integrate cell signaling events with mitochondrial dynamics.
Figures
Figure 1.
Domain structure of yeast Miro (Gem1p). (A) Schematic representation of Gem1p domain structure. Indicated are the predicted GTPase I and II domains, EF-hand I and II motifs, and transmembrane segment (TM). (B) Vertical lines indicate substitutions generated in conserved residues of GTPase I and II domains (Table IV). (C) The consensus CaM (Cmd1p)-like EF-hand motif consists of a helix-loop-helix and is indicated (top). Residues important for calcium coordination are bold. Underlined E in Gem1p(EF I) and Gem1p(EF II) were converted to K for mutational analysis (Table IV). (D) Amino acids 633–652 in the Gem1p COOH terminus are predicted to form a membrane spanning α-helix (Wolff et al., 1999). A similar hydrophobic stretch is present in hMiro-1, other Miro homologues (not depicted), and Fis1p, a tail-anchored outer mitochondrial membrane protein. Predicted transmembrane segments are in italics. Positively charged residues predicted to contribute to mitochondrial membrane targeting are indicated in bold.
Figure 2.
Cells lacking Gem1p grow slowly on synthetic glycerol medium. Strains (JSY7000 GEM1 or JSY7002 _gem1_Δ) with the indicated plasmid were spotted on SDextrose or SGlycerol lacking the appropriate amino acid and grown for 3 d at the indicated temperature. (A) Gem1p expressed from its own promoter (pRS416-GEM1) complements the _gem1_Δ glycerol growth defect. (B) GFP-Gem1p expressed from the TPI promoter (pYX142-GFP-GEM1) partially complements the _gem1_Δ glycerol growth defect.
Figure 3.
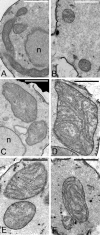
Mitochondrial morphology is abnormal in cells lacking Gem1p. Mitochondrial morphologies are shown for GEM1 cells (A), _gem1_Δ cells (B–D), and fusion-deficient _fzo1_Δ cells (E) expressing mito-GFP and grown in SDextrose medium at 30°C to mid-log phase (OD600 0.5–1.5). GFP fluorescent and DIC images are superimposed for each cell. The percentage of cells in a GEM1 or _gem1_Δ culture with the morphologies depicted in A–E is shown (bottom). n = 900. Bar, 5 μm.
Figure 4.
Inner membrane cristae are present in globular gem1 Δ mitochondria. Mitochondria in wild-type cells (JSY7000) are tubular in longitudinal sections (A) and spherical in cross section (B) and contain inner membrane cristae. Profiles of globular mitochondria in _gem1_Δ strains (JSY7002, C–F), are larger than wild type but contain well-developed cristae. n, nucleus. Bars, 1 μm.
Figure 5.
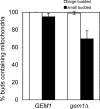
Mitochondrial inheritance is not blocked in the absence of Gem1p. mito-GFP-labeled mitochondria were observed in strains grown to mid-log phase. Large- or small-budded cells containing mitochondria in the bud were scored. n > 250.
Figure 6.
GFP-Gem1p localizes to mitochondria. Wild-type cells (JSY7000) with pYX142-GFP-GEM1 were grown to late log phase (OD600 ∼2) in SDextrose. Mitochondria were stained with MitoFluor red 589. Bar, 5 μm.
Figure 7.
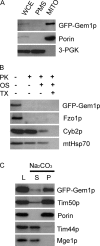
Gem1p is a single-pass outer mitochondrial membrane protein with its NH2 terminus exposed to the cytoplasm. (A) WCE from JSY7000 (GEM1) expressing GFP-Gem1p was fractionated by differential centrifugation to yield PMS and MITO pellet, and analyzed by SDS-PAGE and Western blotting with antibodies against GFP, the multi-pass outer mitochondrial membrane protein Porin, and the cytoplasmic protein 3-PGK. WCE and PMS, 1× cell equivalents; MITO, 10× cell equivalents. (B) Untreated, osmotically shocked (OS), or Triton X-100 solubilized (TX) mitochondria were treated with (+) or without (−) PK and analyzed by SDS-PAGE and Western blotting with antibodies specific for GFP, the integral outer membrane protein Fzo1p, the intermembrane space protein Cyb2p, and the matrix protein mtHsp70. (C) Mitochondria (200 μg) were treated with sodium carbonate (pH 11.5) and separated into supernatant (S) and pellet (P) fractions. One-sixth of the reaction was loaded in each lane (L, load, untreated mitochondria). Tim50p is a single-pass inner membrane protein. Tim44p is a peripheral membrane protein in the matrix. Mge1p is a soluble matrix protein.
Figure 8.
The COOH-terminal 45 aa of Gem1p are sufficient for mitochondrial targeting. Images of GEM1 cells (JSY7000) expressing GFP alone (A–D) or GFP-Gem1p (aa618-662) (E–H) are shown. Mitochondria were visualized simultaneously with mito-RFP. v, vacuole. Bar, 5 μm.
Figure 9.
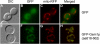
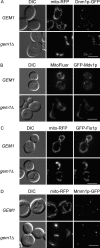
Previously characterized mitochondrial morphology proteins are properly localized in gem1 Δ cells. Colocalization of mitochondria and GFP-labeled Dnm1p (A), Mdv1p (B), Fis1p (C), and Mmm1p (D) was observed in log phase GEM1 and _gem1_Δ cells. Bars, 5 μm.
Figure 10.
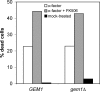
Gem1p is not required for pheromone-induced cell death. Cell death was induced by treating log phase cultures with 20 μM α-factor in the presence/absence of the calcineurin inhibitor FK506 (2 μg/ml). Dead cells were visualized after 10 h by methylene blue staining.
Similar articles
- Structure-function analysis of the yeast mitochondrial Rho GTPase, Gem1p: implications for mitochondrial inheritance.
Koshiba T, Holman HA, Kubara K, Yasukawa K, Kawabata S, Okamoto K, MacFarlane J, Shaw JM. Koshiba T, et al. J Biol Chem. 2011 Jan 7;286(1):354-62. doi: 10.1074/jbc.M110.180034. Epub 2010 Oct 29. J Biol Chem. 2011. PMID: 21036903 Free PMC article. - A hunt for OM45 synthetic petite interactions in Saccharomyces cerevisiae reveals a role for Miro GTPase Gem1p in cristae structure maintenance.
Shvetsova A, Masud AJ, Schneider L, Bergmann U, Monteuuis G, Miinalainen IJ, Hiltunen JK, Kastaniotis AJ. Shvetsova A, et al. Microbiologyopen. 2021 Oct;10(5):e1238. doi: 10.1002/mbo3.1238. Microbiologyopen. 2021. PMID: 34713605 Free PMC article. - Ugo1p links the Fzo1p and Mgm1p GTPases for mitochondrial fusion.
Sesaki H, Jensen RE. Sesaki H, et al. J Biol Chem. 2004 Jul 2;279(27):28298-303. doi: 10.1074/jbc.M401363200. Epub 2004 Apr 14. J Biol Chem. 2004. PMID: 15087460 - Mitochondrial dynamics in yeast.
Hermann GJ, Shaw JM. Hermann GJ, et al. Annu Rev Cell Dev Biol. 1998;14:265-303. doi: 10.1146/annurev.cellbio.14.1.265. Annu Rev Cell Dev Biol. 1998. PMID: 9891785 Review. - The ERMES complex and ER-mitochondria connections.
Michel AH, Kornmann B. Michel AH, et al. Biochem Soc Trans. 2012 Apr;40(2):445-50. doi: 10.1042/BST20110758. Biochem Soc Trans. 2012. PMID: 22435828 Review.
Cited by
- Dramatic changes in mitochondrial subcellular location and morphology accompany activation of the CO2 concentrating mechanism.
Findinier J, Joubert LM, Fakhimi N, Schmid MF, Malkovskiy AV, Chiu W, Burlacot A, Grossman AR. Findinier J, et al. Proc Natl Acad Sci U S A. 2024 Oct 22;121(43):e2407548121. doi: 10.1073/pnas.2407548121. Epub 2024 Oct 15. Proc Natl Acad Sci U S A. 2024. PMID: 39405346 - Miro GTPases at the Crossroads of Cytoskeletal Dynamics and Mitochondrial Trafficking.
Aspenström P. Aspenström P. Cells. 2024 Apr 7;13(7):647. doi: 10.3390/cells13070647. Cells. 2024. PMID: 38607086 Free PMC article. Review. - Dramatic Changes in Mitochondrial Subcellular Location and Morphology Accompany Activation of the CO2 Concentrating Mechanism.
Findinier J, Joubert LM, Schmid MF, Malkovskiy A, Chiu W, Burlacot A, Grossman AR. Findinier J, et al. bioRxiv [Preprint]. 2024 Mar 27:2024.03.25.586705. doi: 10.1101/2024.03.25.586705. bioRxiv. 2024. PMID: 38585955 Free PMC article. Updated. Preprint. - Mitochondria-Endoplasmic Reticulum Contact Sites (MERCS): A New Axis in Neuronal Degeneration and Regeneration.
Sathyamurthy VH, Nagarajan Y, Parvathi VD. Sathyamurthy VH, et al. Mol Neurobiol. 2024 Sep;61(9):6528-6538. doi: 10.1007/s12035-024-03971-6. Epub 2024 Feb 6. Mol Neurobiol. 2024. PMID: 38321352 Review. - Mitochondrial transport in neurons and evidence for its involvement in acute neurological disorders.
Lu D, Feng Y, Liu G, Yang Y, Ren Y, Chen Z, Sun X, Guan Y, Wang Z. Lu D, et al. Front Neurosci. 2023 Oct 12;17:1268883. doi: 10.3389/fnins.2023.1268883. eCollection 2023. Front Neurosci. 2023. PMID: 37901436 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM053466/GM/NIGMS NIH HHS/United States
- 5P30 CA 42014/CA/NCI NIH HHS/United States
- S10 RR019409-01/RR/NCRR NIH HHS/United States
- GM 53466/GM/NIGMS NIH HHS/United States
- P30 CA042014/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases