Mechanisms of human papillomavirus-induced oncogenesis - PubMed (original) (raw)
Review
Mechanisms of human papillomavirus-induced oncogenesis
Karl Münger et al. J Virol. 2004 Nov.
No abstract available
Figures
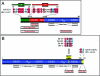
FIG. 1.
(A) Schematic representation of the HPV-16 double-stranded circular DNA genome. The early (E) and late (L) genes, as well as the LCR, are shown. The major early promoter (P97) is indicated by an arrow. Transcription occurs from one strand only and is in clockwise orientation in this representation. See the text for details. (B) Schematic structure of the minimal HPV-16 genome fragment (red) retained after integration into a host chromosome (blue). The HPV E6/E7 genes are consistently expressed, whereas the remaining HPV genes are often deleted or not transcribed after integration. Two major HPV RNA species are produced. One transcript has the potential to encode full-length E6 and E7 proteins, and another set of transcripts encodes spliced E6 proteins (designated E6*) and the full-length E7 protein. Most HPV transcripts in cervical cancer cells are spliced downstream of the E7 gene and use cellular splicing and polyadenylation signals. This may cause increased stability of HPV transcripts. See the text for details.
FIG. 2.
(A) Schematic representation of the HPV-16 E7 oncoprotein. The amino-terminal 37 amino acid residues have sequence similarity to a portion of CR1 (green) and to CR2 (red) of Ad E1A. Identical and chemically similar amino acid residues between HPV-16 E7 and Ad5 E1A are highlighted by red and blue boxes, respectively. CR1 sequences are necessary for cellular transformation and pRB degradation but do not directly contribute to pRB binding. Sequences in CR2 include the core pRB binding site (LXCXE), which is necessary for cellular transformation, as well as a casein kinase II consensus phosphorylation site (CKII). The E7 carboxyl terminus (blue) contains a metal binding motif and mediates association with multiple host cellular proteins, including histone-modifying enzymes, which may also contribute to cellular transformation. See the text for details and references. (B) Schematic representation of the HPV-16 E6 oncoprotein. The sequence contains two metal binding motifs that are related to the E7 carboxyl terminus (blue). The E6 carboxyl terminus contains a PDZ protein-binding motif (yellow) that is similar to the carboxyl-terminal PDZ binding motif of Ad9 E4 ORF1. Many HPV-16 E6 binding proteins, including E6-AP, paxillin, E6-BP, and IRF-3, contain a conserved α-helical domain and presumably interact with similar E6 sequences. The isoleucine residue at position 128 importantly contributes to interaction with α-helix domains containing E6 binding proteins. Identical and chemically similar amino acid residues are highlighted by red and blue boxes, respectively. See the text for details and references.
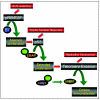
FIG. 3.
Schematic outline of critical steps of high-risk HPV-induced carcinogenesis. Inactivation of the pRB and p53 tumor suppressor pathways and expression of the catalytic telomerase subunit hTERT constitute a subset of the steps that have been shown to be necessary for the generation of fully transformed human epithelial cells in vitro. See the text for details.
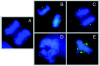
FIG. 4.
The HPV-16 E7 oncoprotein contributes to induction of genomic instability by induction of centrosome duplication errors. Shown are examples of different mitotic abnormalities that can be generated by numerical centrosome abnormalities. (A) Normal bipolar metaphase; each mitotic spindle pole body consists of a single centrosome which contains two centrioles. Individual centrioles are visualized by green fluorescent protein (GFP)-centrin fluorescence. (B) Abnormal bipolar mitosis due to centrosome aggregation. Individual centrioles are visualized by GFP-centrin fluorescence. The mitotic spindle pole on the left contains three centrioles, whereas the one on right contains four centrioles that may represent two aggregated centrosomes. There is a chance for nonsymmetrical chromosome segregation upon completion of cell division. (C) Abnormal bipolar mitosis in the presence of multiple individual centrosomes. Individual centrioles are visualized by immunofluorescence by using a centrin-specific antibody. While the majority of the chromosomes are segregated in a bipolar fashion, the centrosomes on the left may interfere with symmetrical chromosome distribution by apparently capturing some chromosomal material. (D) Predominantly monopolar mitosis in the presence of multiple centrosomes. Individual centrosomes are visualized by immunofluorescence by using a γ-tubulin-specific antibody. (E) Tripolar mitotic figures are hallmarks of high-risk HPV-associated cervical lesions. Individual centrosomes are visualized by GFP-γ-tubulin fluorescence.
Similar articles
- Mechanisms of genomic instability in human cancer: insights from studies with human papillomavirus oncoproteins.
Duensing S, Münger K. Duensing S, et al. Int J Cancer. 2004 Mar 20;109(2):157-62. doi: 10.1002/ijc.11691. Int J Cancer. 2004. PMID: 14750163 Review. - Centrosome-mediated chromosomal instability and steroid hormones as co factors in human papillomavirus-associated cervical carcinogenesis: small viruses help to answer big questions.
Duensing A, Duensing S. Duensing A, et al. Adv Exp Med Biol. 2008;617:109-17. doi: 10.1007/978-0-387-69080-3_10. Adv Exp Med Biol. 2008. PMID: 18497035 Review. No abstract available. - [Current advances in the mechanic studies of human papillomavirus-induced oncogenesis].
Zhou XB, Xu NZ. Zhou XB, et al. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2007 Oct;29(5):673-7. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2007. PMID: 18051727 Review. Chinese. - Human papillomavirus type 18 variants: histopathology and E6/E7 polymorphisms in three countries.
De Boer MA, Peters LA, Aziz MF, Siregar B, Cornain S, Vrede MA, Jordanova ES, Fleuren GJ. De Boer MA, et al. Int J Cancer. 2005 Apr 10;114(3):422-5. doi: 10.1002/ijc.20727. Int J Cancer. 2005. PMID: 15551313
Cited by
- Whole Genomic Analysis and Comparison of Two Canine Papillomavirus Type 9 Strains in Malignant and Benign Skin Lesions.
Chang CY, Yamashita-Kawanishi N, Tomizawa S, Liu IL, Chen WT, Chang YC, Huang WH, Tsai PS, Shirota K, Chambers JK, Uchida K, Haga T, Chang HW. Chang CY, et al. Viruses. 2020 Jul 8;12(7):736. doi: 10.3390/v12070736. Viruses. 2020. PMID: 32650357 Free PMC article. - Interaction between the human papillomavirus 16 E7 oncoprotein and gelsolin ignites cancer cell motility and invasiveness.
Matarrese P, Abbruzzese C, Mileo AM, Vona R, Ascione B, Visca P, Rollo F, Benevolo M, Malorni W, Paggi MG. Matarrese P, et al. Oncotarget. 2016 Aug 9;7(32):50972-50985. doi: 10.18632/oncotarget.8646. Oncotarget. 2016. PMID: 27072581 Free PMC article. - Structure of the p53 degradation complex from HPV16.
Wang JCK, Baddock HT, Mafi A, Foe IT, Bratkowski M, Lin TY, Jensvold ZD, Preciado López M, Stokoe D, Eaton D, Hao Q, Nile AH. Wang JCK, et al. Nat Commun. 2024 Feb 28;15(1):1842. doi: 10.1038/s41467-024-45920-w. Nat Commun. 2024. PMID: 38418456 Free PMC article. - Notch Signaling and Human Papillomavirus-Associated Oral Tumorigenesis.
Das T, Zhong R, Spiotto MT. Das T, et al. Adv Exp Med Biol. 2021;1287:105-122. doi: 10.1007/978-3-030-55031-8_8. Adv Exp Med Biol. 2021. PMID: 33034029 Free PMC article. Review. - Insights into the mechanisms and structure of breakage-fusion-bridge cycles in cervical cancer using long-read sequencing.
Rodriguez I, Rossi NM, Keskus AG, Xie Y, Ahmad T, Bryant A, Lou H, Paredes JG, Milano R, Rao N, Tulsyan S, Boland JF, Luo W, Liu J, O'Hanlon T, Bess J, Mukhina V, Gaykalova D, Yuki Y, Malik L, Billingsley KJ, Blauwendraat C, Carrington M, Yeager M, Mirabello L, Kolmogorov M, Dean M. Rodriguez I, et al. Am J Hum Genet. 2024 Mar 7;111(3):544-561. doi: 10.1016/j.ajhg.2024.01.002. Epub 2024 Feb 1. Am J Hum Genet. 2024. PMID: 38307027 Free PMC article.
References
- Alazawi, W., M. Pett, B. Arch, L. Scott, T. Freeman, M. A. Stanley, and N. Coleman. 2002. Changes in cervical keratinocyte gene expression associated with integration of human papillomavirus 16. Cancer Res. 62:6959-6965. - PubMed
- Androphy, E. J., I. Dvoretzky, and D. R. Lowy. 1985. X-linked inheritance of epidermodysplasia verruciformis. Genetic and virologic studies of a kindred. Arch. Dermatol. 121:864-868. - PubMed
- Avvakumov, N., J. Torchia, and J. S. Mymryk. 2003. Interaction of the HPV E7 proteins with the pCAF acetyltransferase. Oncogene 22:3833-3841. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA81135/CA/NCI NIH HHS/United States
- DE015302/DE/NIDCR NIH HHS/United States
- R01 CA081135/CA/NCI NIH HHS/United States
- R01 DE015302/DE/NIDCR NIH HHS/United States
- T32CA09031/CA/NCI NIH HHS/United States
- CA66980/CA/NCI NIH HHS/United States
- T32 CA009031/CA/NCI NIH HHS/United States
- R01 CA066980/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical