Evidence that DeltaNp73 promotes neuronal survival by p53-dependent and p53-independent mechanisms - PubMed (original) (raw)
Evidence that DeltaNp73 promotes neuronal survival by p53-dependent and p53-independent mechanisms
Anna F Lee et al. J Neurosci. 2004.
Abstract
The p53 family member, p73, is essential for the survival of sympathetic neurons during the developmental period of naturally occurring neuronal death. Here, we have asked whether DeltaNp73, which is the only p73 isoform expressed in sympathetic neurons, mediates this survival by p53-dependent and/or p53-independent mechanisms. Initially, we used a genetic approach and crossed p53+/- and p73+/- mice. Quantitation of neurons in the sympathetic superior cervical ganglion during the period of naturally occurring cell death revealed that the loss of p53 partially rescued the death of neurons seen in p73-/- animals. Moreover, exogenous expression of DeltaNp73 in cultured p53-/- sympathetic neurons rescued these neurons from apoptosis after NGF withdrawal. Biochemical studies asking how DeltaNp73 inhibited NGF withdrawal-induced apoptosis in wild-type neurons demonstrated that it prevented the upregulation of the direct p53 targets p21 and Apaf-1 as well as cleavage of caspase-3. It also inhibited events at the mitochondrial apoptotic checkpoint, suppressing the induction of BimEL and the release of mitochondrial cytochrome c. Interestingly, DeltaNp73 expression also inhibited one very early event in the apoptotic cascade, the activation of c-Jun N-terminal protein kinase (JNK), likely by binding directly to JNK. Finally, we show that neuronal cell size is decreased in p73-/- mice, and that this decrease is not rescued by the lack of p53, suggesting a role for p73 in regulating cell size that does not involve interactions with p53. Thus, DeltaNp73 promotes neuronal survival via p53-dependent and -independent mechanisms, and it does so at multiple points, including some of the most proximal events that occur after NGF withdrawal.
Figures
Figure 1.
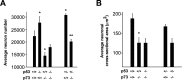
The coincident absence of p53 partially rescues the phenotype of the p73-/- sympathetic superior cervical ganglion. A, Photomicrographs of cryosections of SCGs isolated from P10 progeny of different genotypes deriving from crosses of p53+/-, p73+/- animals. Representative cross-sections from the center of the SCGs were stained with cresyl violet and photographed at the same magnification. Scale bar, 100 μm. B, Photomicrographs of neurons within cryosections similar to those shown in A. The arrowheads indicate single neurons. Scale bar, 25 μm. C, Immunocytochemical analysis for TH in sections of the SCG from a p53+/-, p73-/- animal to demonstrate that sympathetic neurons have an appropriate noradrenergic phenotype in the absence of p73. The arrowheads indicate TH-positive neurons.
Figure 2.
Loss of p53 partially rescues the loss of sympathetic neurons in p73-/- ganglia. A, Quantitation of the mean sympathetic neuron number in P10 SCGs of different genotypes deriving from crosses of p53+/-, p73+/- animals. With the exception of p53-/-, p73-/- genotype, the bars represent the mean neuron numbers ± SEM (n > 3). For the p53-/-, p73-/- neurons, the bar indicates the average neuron number of two SCGs ± SEM (*p < 0.05 relative to p53+/+, p73+/+; **p < 0.05 relative to p53+/+, p73-/-). B, Quantitation of sympathetic neuron size in P10 SCGs of the progeny of p53+/-, p73+/- crosses. Loss of p53 does not rescue the reduced neuron size seen in p73-/- mice (*p < 0.05 relative to p53+/+, p73+/+)
Figure 3.
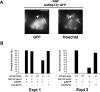
ΔNp73 rescues p53-/- neurons from NGF withdrawal-induced apoptosis. A, Fluorescence micrographs of p53-/- sympathetic neurons infected with a recombinant adenovirus expressing both ΔNp73β and GFP (Ad5ΔNp73β·GFP) withdrawn from NGF for 48 hr and then counterstained with Hoechst to show the nuclear morphology. The arrowhead indicates an uninfected neuron with a pyknotic, apoptotic nucleus, whereas the arrow denotes one of several infected GFP-positive neurons in the field that show round, healthy nuclei. B, Bar graphs showing quantitation of two representative experiments (of 3 in total) similar to that shown in A. p53+/+ and p53-/- neurons cultured from neonatal littermates were infected either with an adenovirus expressing only GFP (Ad5GFP) or with one expressing both ΔNp73β and GFP (Ad5ΔNp73β) withdrawn from NGF for 48 hr and analyzed as in A. Scale bar, 50 μm. Data were normalized so that the survival of GFP-infected p53+/+ neurons in NGF was 100%, and GFP-infected p53+/+ neurons withdrawn from NGF for 48 hr was 0%. Note that ΔNp73β completely rescued p53-/- neurons from apoptosis, and that survival of p53-/- neurons was higher than that of their wild-type counterparts, although the magnitude of this increase was variable.
Figure 4.
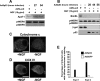
p53 induces increased levels of Apaf-1 as well as cytochrome c release from mitochondria but has no effect on expression of BimEL. A, B, Western blot analysis of cultured neonatal mouse (A) or rat (B) sympathetic neurons that were established in the presence of NGF, transduced with the recombinant adenoviruses Ad5p53 or Ad5LacZ as indicated, and maintained in NGF for various time points after infection. Controls were withdrawn from NGF for 24 hr. Western blots were then probed with antibodies specific for Apaf-1 (A) or BimEL (B). Blots were then reprobed for the ERKs to demonstrate equal loading (p42/p44 ERK). C, Confocal micrographs of sympathetic neurons that were either maintained in NGF (+NGF; left) or withdrawn from NGF for 24 hr (-NGF; right) and then fixed and immunostained for cytochrome c, demonstrating the patterns of staining that were defined as punctate (localized to mitochondria; left) or diffuse (released from mitochondria; right) for quantitation. Scale bar, 10 μm. D, Confocal micrographs of sympathetic neurons that were either maintained in NGF (+NGF; left) or withdrawn from NGF (-NGF; right) on DIV 5 and then immunostained 24 hr later for COX IV, a mitochondrial marker. Note that COX IV staining remains bright and punctate after NGF withdrawal. E, Quantitation of diffuse cytochrome c distribution, indicative of cytochrome c that has been released from mitochondria in sympathetic neurons 48 hr after infection with recombinant adenoviruses expressing either GFP or p53. Infected neurons were fixed and immunostained for p53 and cytochrome c, nuclei were visualized by Hoechstdye, and the percentage of infected neurons showing diffuse cytochrome c immunostaining was determined. Data represent the percentage of infected cells with diffuse cytochrome c staining in two independent experiments.
Figure 6.
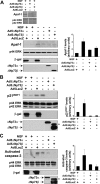
ΔNp73 prevents cytochrome c release from mitochondria and BimEL upregulation in sympathetic neurons after NGF withdrawal. A, Quantitation of cytochrome c distribution at 24 hr post-NGF withdrawal in established cultures of neonatal rat sympathetic neurons that were infected with either LacZ or ΔNp73β adenovirus, the latter of which coexpresses GFP, and withdrawn from NGF on DIV5. After 24 hr, cells were fixed and immunostained for β-galactosidase and cytochrome c (for Ad 5 LacZ) or for GFP and cytochrome c (for Ad5ΔNp73β). Nuclei were visualized by Hoechst dye, and the percentage of infected neurons showing punctate (black bars) versus diffuse (white bars) cytochrome c immunostaining was determined. Error bars indicate the mean ± SEM (n = 3 independent experiments). B, Western blot analysis for BimEL in cultured neonatal rat sympathetic neurons that were established for 3 DIV, infected with Ad5LacZ, Ad5ΔNp73α, or Ad5ΔNp73β, withdrawn from NGF on DIV5, and then analyzed 20 hr later. As a control, neurons were maintained in NGF for the entirety of the experiment. The positive controls are lysates of cultured human embryonic kidney 293 cells or HeLa cells. The blots were reprobed for the ERK proteins to ensure equal loading of protein (p44 ERK), and adenoviral gene transfer was confirmed by probing the same lysates with antibodies specific for β-galactosidase and p73. These blots are representative of those obtained in three independent experiments. The fold-induction of BimEL from two independent experiments is also graphed, demonstrating the consistency of the rescue with ΔNp73. The asterisk (*) in the p73 reprobe denotes a nonspecific band. A background band (**) is present because of incomplete stripping of the ERK blot.
Figure 5.
ΔNp73 blocks the increases in p21WAF1 and Apaf-1 and cleavage of caspase-3 in sympathetic neurons withdrawn from NGF. A-C, Western blot analysis of cultured neonatal mouse (A) or rat (B, C) sympathetic neurons that were established in the presence of NGF, transduced with the recombinant adenoviruses Ad5ΔNp73α, Ad5ΔNp73β, or Ad5LacZ as indicated, withdrawn from NGF on DIV5, and lysed 24 hr later. Controls were maintained in NGF for the entirety of the experiment. Western blots were then probed with antibodies specific for Apaf-1 (A), for p21WAF1 (B), or for cleaved activated caspase-3 (C). All blots were then reprobed for the ERKs to demonstrate equal loading (p42/p44 ERK). Adenoviral gene transfer was confirmed by performing Western blots on the same lysates with antibodies specific for β-galactosidase and for p73. The average fold-induction ± SEM of the relevant protein from two independent experiments is also graphed, demonstrating the consistency of the rescue with ΔNp73. The asterisk (*) in the p73 reprobe denotes a nonspecific band.
Figure 7.
A, B, ΔNp73 binds to JNK and inhibits activation of JNK, an early apoptotic signaling event after NGF withdrawal of sympathetic neurons. A, B, Established cultures of neonatal rat sympathetic neurons were infected with Ad5LacZ, Ad5ΔNp73α, or Ad5ΔNp73β adenovirus, withdrawn from NGF after 5 DIV and then analyzed 8 hr later. A, Western blot analysis of neurons treated as indicated and then probed with an antibody specific to the phosphorylated, activated form of JNKs 1 and 2 (P-p46/p54 JNK). The blot was then reprobed first for total JNK protein (p46/p54 JNK) and then for ERK protein (p42/p44 ERK) to ensure that equal amounts of protein were loaded in each lane. Adenoviral gene transfer was confirmed by probing the same lysates with antibodies specific for β-galactosidase and p73. The fold induction of phosphorylated JNK from a second independent experiment is also graphed, demonstrating the consistency of the rescue with ΔNp73. The asterisk (*) in the p73 reprobe denotes a nonspecific band. B, In vitro JNK activity assay of neurons treated as indicated, using GST-c-jun (1-169) as a substrate (arrow). Autophosphorylated JNK is marked with an asterisk (*). The blot was then probed for JNK to visualize the amounts of immunoprecipitated JNK (p46 JNK). The IgG heavy chain (IgG) is also present. C-E, ΔNp73 binds directly to JNK. C, Lysates of cultured cortical neurons were incubated with a GST-ΔNp73β fusion protein or as a control with GST alone, and the GST fusion protein was then precipitated with glutathione Sepharose beads. Precipitates were analyzed on Western blots, and the blots were probed with an antibody specific to JNK. The input cortical neuron lysate is also shown for comparison. Note that JNK is present in the GST-ΔNp73β precipitate but not in the GST-alone precipitates. D, Cortical neurons were infected with recombinant adenoviruses expressing either ΔNp73α orβ, and nuclear fractions were immunoprecipitated with an anti-p73 antibody. Immunoprecipitates were analyzed by Western blot analysis with an antibody specific to JNK (p46 JNK). The blots were reprobed with an antibody specific for p73 (bottom). The input cortical neuron lysate is also shown for comparison. Note that JNK associates with both ΔNp73 isoforms. E, Purified, active, phosphorylated JNKα1 or inactive, nonphosphorylated JNKα1 was mixed with GST-ΔNp73β bound to glutathione Sepharose beads. The beads were then precipitated and analyzed on Western blots. As a positive control, aliquots of the mixture containing active or inactive JNKα1 were run on the same blot. Note that JNKα1 is present in the GST-ΔNp73β precipitates but not in the GST-alone precipitates.
Similar articles
- The invulnerability of adult neurons: a critical role for p73.
Walsh GS, Orike N, Kaplan DR, Miller FD. Walsh GS, et al. J Neurosci. 2004 Oct 27;24(43):9638-47. doi: 10.1523/JNEUROSCI.1299-04.2004. J Neurosci. 2004. PMID: 15509751 Free PMC article. - Dissociation of JNK Activation from Elevated Levels of Reactive Oxygen Species, Cytochrome c Release, and Cell Death in NGF-Deprived Sympathetic Neurons.
McManus MJ, Franklin JL. McManus MJ, et al. Mol Neurobiol. 2018 Jan;55(1):382-389. doi: 10.1007/s12035-016-0332-2. Epub 2016 Dec 12. Mol Neurobiol. 2018. PMID: 27957682 - p73 Induces apoptosis via PUMA transactivation and Bax mitochondrial translocation.
Melino G, Bernassola F, Ranalli M, Yee K, Zong WX, Corazzari M, Knight RA, Green DR, Thompson C, Vousden KH. Melino G, et al. J Biol Chem. 2004 Feb 27;279(9):8076-83. doi: 10.1074/jbc.M307469200. Epub 2003 Nov 21. J Biol Chem. 2004. PMID: 14634023 - Negative autoregulation of p73 and p53 by DeltaNp73 in regulating differentiation and survival of human neuroblastoma cells.
Nakagawa T, Takahashi M, Ozaki T, Watanabe K, Hayashi S, Hosoda M, Todo S, Nakagawara A. Nakagawa T, et al. Cancer Lett. 2003 Jul 18;197(1-2):105-9. doi: 10.1016/s0304-3835(03)00090-9. Cancer Lett. 2003. PMID: 12880968 Review. - No more brain tangles with DeltaNp73.
Mattson MP, Ashery U. Mattson MP, et al. Trends Biochem Sci. 2009 Jan;34(1):6-8. doi: 10.1016/j.tibs.2008.10.004. Epub 2008 Nov 13. Trends Biochem Sci. 2009. PMID: 19008105 Free PMC article. Review.
Cited by
- Complex regulation of p73 isoforms after alteration of amyloid precursor polypeptide (APP) function and DNA damage in neurons.
Benosman S, Meng X, Von Grabowiecki Y, Palamiuc L, Hritcu L, Gross I, Mellitzer G, Taya Y, Loeffler JP, Gaiddon C. Benosman S, et al. J Biol Chem. 2011 Dec 16;286(50):43013-25. doi: 10.1074/jbc.M111.261271. Epub 2011 Oct 14. J Biol Chem. 2011. PMID: 22002055 Free PMC article. - Conditional ablation of p63 indicates that it is essential for embryonic development of the central nervous system.
Cancino GI, Fatt MP, Miller FD, Kaplan DR. Cancino GI, et al. Cell Cycle. 2015;14(20):3270-81. doi: 10.1080/15384101.2015.1087618. Cell Cycle. 2015. PMID: 26359534 Free PMC article. - ΔNp73/ETS2 complex drives glioblastoma pathogenesis- targeting downstream mediators by rebastinib prolongs survival in preclinical models of glioblastoma.
Cam M, Charan M, Welker AM, Dravid P, Studebaker AW, Leonard JR, Pierson CR, Nakano I, Beattie CE, Hwang EI, Kambhampati M, Nazarian J, Finlay JL, Cam H. Cam M, et al. Neuro Oncol. 2020 Mar 5;22(3):345-356. doi: 10.1093/neuonc/noz190. Neuro Oncol. 2020. PMID: 31763674 Free PMC article. - Pathogenic Effect of TP73 Gene Variants in People With Amyotrophic Lateral Sclerosis.
Russell KL, Downie JM, Gibson SB, Tsetsou S, Keefe MD, Duran JA, Figueroa KP, Bromberg MB, Murtaugh LC, Bonkowsky JL, Pulst SM, Jorde LB. Russell KL, et al. Neurology. 2021 Jul 19;97(3):e225-e235. doi: 10.1212/WNL.0000000000012285. Neurology. 2021. PMID: 34135078 Free PMC article. - The invulnerability of adult neurons: a critical role for p73.
Walsh GS, Orike N, Kaplan DR, Miller FD. Walsh GS, et al. J Neurosci. 2004 Oct 27;24(43):9638-47. doi: 10.1523/JNEUROSCI.1299-04.2004. J Neurosci. 2004. PMID: 15509751 Free PMC article.
References
- Armstrong JF, Kaufman MH, Harrison DJ, Clarke AR (1995) High-frequency developmental abnormalities in p53-deficient mice. Curr Biol 5: 931-936. - PubMed
- Backman SA, Stambolic V, Suzuki A, Haight J, Elia A, Pretorius J, Tsao MS, Shannon P, Bolon B, Ivy GO, Mak TW (2001) Deletion of Pten in mouse brain causes seizures, ataxia and defects in soma size resembling Lhermitte-Duclos disease. Nat Genet 29: 396-403. - PubMed
- Besirli CG, Deckwerth TL, Crowder RJ, Freeman RS, Johnson Jr EM (2003) Cytosine arabinoside rapidly activates Bax-dependent apoptosis and a delayed Bax-independent death pathway in sympathetic neurons. Cell Death Differ 10: 1045-1058. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous