DNA damage-induced cell cycle checkpoint control requires CtIP, a phosphorylation-dependent binding partner of BRCA1 C-terminal domains - PubMed (original) (raw)
DNA damage-induced cell cycle checkpoint control requires CtIP, a phosphorylation-dependent binding partner of BRCA1 C-terminal domains
Xiaochun Yu et al. Mol Cell Biol. 2004 Nov.
Abstract
The BRCA1 C-terminal (BRCT) domain has recently been implicated as a phospho-protein binding domain. We demonstrate here that a CTBP-interacting protein CtIP interacts with BRCA1 BRCT domains in a phosphorylation-dependent manner. The CtIP/BRCA1 complex only exists in G(2) phase and is required for DNA damage-induced Chk1 phosphorylation and the G(2)/M transition checkpoint. However, the CtIP/BRCA1 complex is not required for the damage-induced G(2) accumulation checkpoint, which is controlled by a separate BRCA1/BACH1 complex. Taken together, these data not only implicate CtIP as a critical player in cell cycle checkpoint control but also provide molecular mechanisms by which BRCA1 controls multiple cell cycle transitions after DNA damage.
Figures
FIG. 1.
The BRCA1 BRCT domain interacts specifically with phosphorylated CtIP. (A) Flag-tagged CtIP deletion mutants were expressed in 293T cells. Cell lysates were incubated with GST-BRCA1-BRCT protein immobilized on beads. Associated CtIP proteins were eluted and detected with anti-Flag antibody (upper panels). Whole-cell lysates were also blotted with anti-Flag antibody to ensure similar expression levels of various CtIP mutants (lower panels). (B) Potential phospho-motif on CtIP. (C to E) Wild-type or S327A mutant of CtIP was expressed in 293T cells. Cell lysates were incubated with beads containing GST-BRCA1-BRCT for pull-down assay (C) or incubated with the indicated antibodies for immunoprecipitation (D and E). Associated or immunoprecipitated proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted with indicated antibodies. (F) Endogenous CtIP from 293T cell lysate was immunoprecipitated with anti-CtIP antibody, followed by treatment with or without λ phosphatase (PPase) or phosphatase inhibitor. Immunoprecipitated CtIP was then immunoblotted with anti-phospho-S327 antibody (upper panels). An identical blot was also blotted with anti-CtIP antibody to ensure similar amounts of CtIP in these experiments. (G) Phosphorylated CtIP peptide competes with endogenous CtIP for BRCA1 binding. 293T cell lysates were incubated with beads containing GST-BRCA1-BRCT proteins in the presence of 10 μM phosphorylated peptide (pS, TRVSpSPVFGAT) or unphosphorylated control peptide (S, TRVSSPVFGAT). Endogenous CtIP associated with BRCA1 BRCT domains was eluted and detected by using anti-CtIP antibody.
FIG. 1.
The BRCA1 BRCT domain interacts specifically with phosphorylated CtIP. (A) Flag-tagged CtIP deletion mutants were expressed in 293T cells. Cell lysates were incubated with GST-BRCA1-BRCT protein immobilized on beads. Associated CtIP proteins were eluted and detected with anti-Flag antibody (upper panels). Whole-cell lysates were also blotted with anti-Flag antibody to ensure similar expression levels of various CtIP mutants (lower panels). (B) Potential phospho-motif on CtIP. (C to E) Wild-type or S327A mutant of CtIP was expressed in 293T cells. Cell lysates were incubated with beads containing GST-BRCA1-BRCT for pull-down assay (C) or incubated with the indicated antibodies for immunoprecipitation (D and E). Associated or immunoprecipitated proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted with indicated antibodies. (F) Endogenous CtIP from 293T cell lysate was immunoprecipitated with anti-CtIP antibody, followed by treatment with or without λ phosphatase (PPase) or phosphatase inhibitor. Immunoprecipitated CtIP was then immunoblotted with anti-phospho-S327 antibody (upper panels). An identical blot was also blotted with anti-CtIP antibody to ensure similar amounts of CtIP in these experiments. (G) Phosphorylated CtIP peptide competes with endogenous CtIP for BRCA1 binding. 293T cell lysates were incubated with beads containing GST-BRCA1-BRCT proteins in the presence of 10 μM phosphorylated peptide (pS, TRVSpSPVFGAT) or unphosphorylated control peptide (S, TRVSSPVFGAT). Endogenous CtIP associated with BRCA1 BRCT domains was eluted and detected by using anti-CtIP antibody.
FIG. 2.
Phosphorylation-dependent formation of BRCA1/CtIP complex is cell cycle regulated. (A and B) HeLa cells were arrested in M phase by treatment with nocodazole (1 μg/ml) for 24 h (A) or at G1/S boundary by double thymidine block (B). Cells were then released and harvested at the indicated time points. Immunoprecipitations and immunoblotting were performed as indicated. Since Chk2 protein level is not cell cycle regulated, an anti-Chk2 immunoblot was included as a protein loading control. Both anti-cyclin B and anti-phospho-histone H3 blots were used as controls for cell cycle progress. Cell cycle distributions were analyzed by fluorescence-activated cell sorting and summarized here.
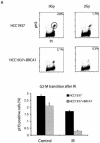
FIG. 3.
Phosphorylation-dependent BRCA1 complexes participate in different cell cycle checkpoints after DNA damage. (A and B) Two checkpoint defects in HCC1937 cells. Checkpoint assays were performed as described in Materials and Methods. The percentage of phospho-histone H3 positive staining cells was measured. (C) HeLa cells were transfected with control, CtIP, BRCA1, or BACH1 siRNA. Cell lysates were immunoblotted with indicated antibodies. Irrelevant siRNA was included as a negative control. G2 accumulation assays were performed 48 h after the initial siRNA transfection. (D) HeLa cells were transfected with control, BRCA1, BACH1, or CtIP siRNA. Transient G2/M transition assays were carried out 48 h after the initial siRNA transfection. (E) siRNA-resistant silent mutant of CtIP (WT) and S327A mutant were expressed in 293T cells by using a lentivirus expression system. Cell lysates were immunoprecipitated with anti-Flag or anti-CtIP antibodies and blotted with anti-CtIP antibody. (F) HeLa cells were transfected with CtIP siRNA after infection with wild type (WT) or S327A lentivirus. Cell lysates were immunoblotted with anti-CtIP or anti-BRCA1 antibodies. Whole-cell lysates (WCL) of untreated cells were included as control. Transient G2/M transition checkpoint assays were performed 48 h after CtIP siRNA transfection.
FIG. 3.
Phosphorylation-dependent BRCA1 complexes participate in different cell cycle checkpoints after DNA damage. (A and B) Two checkpoint defects in HCC1937 cells. Checkpoint assays were performed as described in Materials and Methods. The percentage of phospho-histone H3 positive staining cells was measured. (C) HeLa cells were transfected with control, CtIP, BRCA1, or BACH1 siRNA. Cell lysates were immunoblotted with indicated antibodies. Irrelevant siRNA was included as a negative control. G2 accumulation assays were performed 48 h after the initial siRNA transfection. (D) HeLa cells were transfected with control, BRCA1, BACH1, or CtIP siRNA. Transient G2/M transition assays were carried out 48 h after the initial siRNA transfection. (E) siRNA-resistant silent mutant of CtIP (WT) and S327A mutant were expressed in 293T cells by using a lentivirus expression system. Cell lysates were immunoprecipitated with anti-Flag or anti-CtIP antibodies and blotted with anti-CtIP antibody. (F) HeLa cells were transfected with CtIP siRNA after infection with wild type (WT) or S327A lentivirus. Cell lysates were immunoblotted with anti-CtIP or anti-BRCA1 antibodies. Whole-cell lysates (WCL) of untreated cells were included as control. Transient G2/M transition checkpoint assays were performed 48 h after CtIP siRNA transfection.
FIG. 3.
Phosphorylation-dependent BRCA1 complexes participate in different cell cycle checkpoints after DNA damage. (A and B) Two checkpoint defects in HCC1937 cells. Checkpoint assays were performed as described in Materials and Methods. The percentage of phospho-histone H3 positive staining cells was measured. (C) HeLa cells were transfected with control, CtIP, BRCA1, or BACH1 siRNA. Cell lysates were immunoblotted with indicated antibodies. Irrelevant siRNA was included as a negative control. G2 accumulation assays were performed 48 h after the initial siRNA transfection. (D) HeLa cells were transfected with control, BRCA1, BACH1, or CtIP siRNA. Transient G2/M transition assays were carried out 48 h after the initial siRNA transfection. (E) siRNA-resistant silent mutant of CtIP (WT) and S327A mutant were expressed in 293T cells by using a lentivirus expression system. Cell lysates were immunoprecipitated with anti-Flag or anti-CtIP antibodies and blotted with anti-CtIP antibody. (F) HeLa cells were transfected with CtIP siRNA after infection with wild type (WT) or S327A lentivirus. Cell lysates were immunoblotted with anti-CtIP or anti-BRCA1 antibodies. Whole-cell lysates (WCL) of untreated cells were included as control. Transient G2/M transition checkpoint assays were performed 48 h after CtIP siRNA transfection.
FIG. 3.
Phosphorylation-dependent BRCA1 complexes participate in different cell cycle checkpoints after DNA damage. (A and B) Two checkpoint defects in HCC1937 cells. Checkpoint assays were performed as described in Materials and Methods. The percentage of phospho-histone H3 positive staining cells was measured. (C) HeLa cells were transfected with control, CtIP, BRCA1, or BACH1 siRNA. Cell lysates were immunoblotted with indicated antibodies. Irrelevant siRNA was included as a negative control. G2 accumulation assays were performed 48 h after the initial siRNA transfection. (D) HeLa cells were transfected with control, BRCA1, BACH1, or CtIP siRNA. Transient G2/M transition assays were carried out 48 h after the initial siRNA transfection. (E) siRNA-resistant silent mutant of CtIP (WT) and S327A mutant were expressed in 293T cells by using a lentivirus expression system. Cell lysates were immunoprecipitated with anti-Flag or anti-CtIP antibodies and blotted with anti-CtIP antibody. (F) HeLa cells were transfected with CtIP siRNA after infection with wild type (WT) or S327A lentivirus. Cell lysates were immunoblotted with anti-CtIP or anti-BRCA1 antibodies. Whole-cell lysates (WCL) of untreated cells were included as control. Transient G2/M transition checkpoint assays were performed 48 h after CtIP siRNA transfection.
FIG. 3.
Phosphorylation-dependent BRCA1 complexes participate in different cell cycle checkpoints after DNA damage. (A and B) Two checkpoint defects in HCC1937 cells. Checkpoint assays were performed as described in Materials and Methods. The percentage of phospho-histone H3 positive staining cells was measured. (C) HeLa cells were transfected with control, CtIP, BRCA1, or BACH1 siRNA. Cell lysates were immunoblotted with indicated antibodies. Irrelevant siRNA was included as a negative control. G2 accumulation assays were performed 48 h after the initial siRNA transfection. (D) HeLa cells were transfected with control, BRCA1, BACH1, or CtIP siRNA. Transient G2/M transition assays were carried out 48 h after the initial siRNA transfection. (E) siRNA-resistant silent mutant of CtIP (WT) and S327A mutant were expressed in 293T cells by using a lentivirus expression system. Cell lysates were immunoprecipitated with anti-Flag or anti-CtIP antibodies and blotted with anti-CtIP antibody. (F) HeLa cells were transfected with CtIP siRNA after infection with wild type (WT) or S327A lentivirus. Cell lysates were immunoblotted with anti-CtIP or anti-BRCA1 antibodies. Whole-cell lysates (WCL) of untreated cells were included as control. Transient G2/M transition checkpoint assays were performed 48 h after CtIP siRNA transfection.
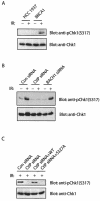
FIG. 4.
CtIP is required for Chk1 activation after DNA damage. (A) HCC1937 cells or HCC1937-BRCA1 cells were treated with or without irradiation (IR; 8 Gy). Cell lysates were collected and immunoblotted with anti-phospho-Chk1 (anti-pS317) or anti-Chk1 antibodies. (B) HeLa cells were transfected with control, CtIP or BACH1 siRNAs. Cells were treated with or without irradiation (IR; 8 Gy). Cell lysates were collected and immunoblotted with indicated antibodies. (C) HeLa cells were transfected with CtIP siRNA after infection with lentivirus expressing wild type (WT) or the S327A mutant of CtIP. Cells were treated with irradiation (IR; 8 Gy) at 48 h after CtIP siRNA transfection. Cell lysates were immunoblotted with anti-phospho-Chk1 (anti-pS317) or anti-Chk1 antibodies.
Similar articles
- Structural basis for cell cycle checkpoint control by the BRCA1-CtIP complex.
Varma AK, Brown RS, Birrane G, Ladias JA. Varma AK, et al. Biochemistry. 2005 Aug 23;44(33):10941-6. doi: 10.1021/bi0509651. Biochemistry. 2005. PMID: 16101277 - Cell cycle-dependent complex formation of BRCA1.CtIP.MRN is important for DNA double-strand break repair.
Chen L, Nievera CJ, Lee AY, Wu X. Chen L, et al. J Biol Chem. 2008 Mar 21;283(12):7713-20. doi: 10.1074/jbc.M710245200. Epub 2008 Jan 2. J Biol Chem. 2008. PMID: 18171670 - BRCA1 ubiquitinates its phosphorylation-dependent binding partner CtIP.
Yu X, Fu S, Lai M, Baer R, Chen J. Yu X, et al. Genes Dev. 2006 Jul 1;20(13):1721-6. doi: 10.1101/gad.1431006. Genes Dev. 2006. PMID: 16818604 Free PMC article. - CtIP, a candidate tumor susceptibility gene is a team player with luminaries.
Chinnadurai G. Chinnadurai G. Biochim Biophys Acta. 2006 Jan;1765(1):67-73. doi: 10.1016/j.bbcan.2005.09.002. Epub 2005 Oct 6. Biochim Biophys Acta. 2006. PMID: 16249056 Review. - Interactions between BRCT repeats and phosphoproteins: tangled up in two.
Glover JN, Williams RS, Lee MS. Glover JN, et al. Trends Biochem Sci. 2004 Nov;29(11):579-85. doi: 10.1016/j.tibs.2004.09.010. Trends Biochem Sci. 2004. PMID: 15501676 Review.
Cited by
- Paraquat modulates alternative pre-mRNA splicing by modifying the intracellular distribution of SRPK2.
Vivarelli S, Lenzken SC, Ruepp MD, Ranzini F, Maffioletti A, Alvarez R, Mühlemann O, Barabino SM. Vivarelli S, et al. PLoS One. 2013 Apr 16;8(4):e61980. doi: 10.1371/journal.pone.0061980. Print 2013. PLoS One. 2013. PMID: 23613995 Free PMC article. - Phosphorylation of Sae2 Mediates Forkhead-associated (FHA) Domain-specific Interaction and Regulates Its DNA Repair Function.
Liang J, Suhandynata RT, Zhou H. Liang J, et al. J Biol Chem. 2015 Apr 24;290(17):10751-63. doi: 10.1074/jbc.M114.625293. Epub 2015 Mar 11. J Biol Chem. 2015. PMID: 25762720 Free PMC article. - BRCA1 targets G2/M cell cycle proteins for ubiquitination and proteasomal degradation.
Shabbeer S, Omer D, Berneman D, Weitzman O, Alpaugh A, Pietraszkiewicz A, Metsuyanim S, Shainskaya A, Papa MZ, Yarden RI. Shabbeer S, et al. Oncogene. 2013 Oct 17;32(42):5005-16. doi: 10.1038/onc.2012.522. Epub 2012 Dec 17. Oncogene. 2013. PMID: 23246971 Free PMC article. - Sharpening the ends for repair: mechanisms and regulation of DNA resection.
Paudyal SC, You Z. Paudyal SC, et al. Acta Biochim Biophys Sin (Shanghai). 2016 Jul;48(7):647-57. doi: 10.1093/abbs/gmw043. Epub 2016 May 12. Acta Biochim Biophys Sin (Shanghai). 2016. PMID: 27174871 Free PMC article. Review. - Prediction of functional phosphorylation sites by incorporating evolutionary information.
Niu S, Wang Z, Ge D, Zhang G, Li Y. Niu S, et al. Protein Cell. 2012 Sep;3(9):675-90. doi: 10.1007/s13238-012-2048-z. Epub 2012 Jul 16. Protein Cell. 2012. PMID: 22802047 Free PMC article.
References
- Abraham, R. T. 2001. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 15:2177-2196. - PubMed
- Cantor, S. B., D. W. Bell, S. Ganesan, E. M. Kass, R. Drapkin, S. Grossman, D. C. Wahrer, D. C. Sgroi, W. S. Lane, D. A. Haber, and D. M. Livingston. 2001. BACH1, a novel helicase-like protein, interacts directly with BRCA1 and contributes to its DNA repair function. Cell 105:149-160. - PubMed
- Chen, J. 2000. Ataxia telangiectasia-related protein is involved in the phosphorylation of BRCA1 following deoxyribonucleic acid damage. Cancer Res. 60:5037-5039. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous