Nuclear pre-mRNA decapping and 5' degradation in yeast require the Lsm2-8p complex - PubMed (original) (raw)
Nuclear pre-mRNA decapping and 5' degradation in yeast require the Lsm2-8p complex
Joanna Kufel et al. Mol Cell Biol. 2004 Nov.
Abstract
Previous analyses have identified related cytoplasmic Lsm1-7p and nuclear Lsm2-8p complexes. Here we report that mature heat shock and MET mRNAs that are trapped in the nucleus due to a block in mRNA export were strongly stabilized in strains lacking Lsm6p or the nucleus-specific Lsm8p protein but not by the absence of the cytoplasmic Lsm1p. These nucleus-restricted mRNAs remain polyadenylated until their degradation, indicating that nuclear mRNA degradation does not involve the incremental deadenylation that is a key feature of cytoplasmic turnover. Lsm8p can be UV cross-linked to nuclear poly(A)(+) RNA, indicating that an Lsm2-8p complex interacts directly with nucleus-restricted mRNA. Analysis of pre-mRNAs that contain intronic snoRNAs indicates that their 5' degradation is specifically inhibited in strains lacking any of the Lsm2-8p proteins but Lsm1p. Nucleus-restricted mRNAs and pre-mRNA degradation intermediates that accumulate in lsm mutants remain 5' capped. We conclude that the Lsm2-8p complex normally targets nuclear RNA substrates for decapping.
Figures
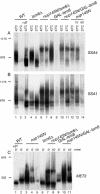
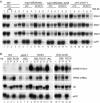
FIG. 1.
Transcriptional shutdown of transiently expressed mRNAs. Northern analysis of MET (A) and heat shock (B) mRNAs. (A) Strains were pregrown in minimal medium lacking methionine at 23°C (23°C lanes), shifted to 37°C for 30 min (37°C 0-min lane), and supplemented with methionine to a 2 mM final concentration. The GAL::lsm8 and nup145N/GAL::lsm8 strains (lanes 37 to 48) were pregrown on galactose and transferred to minimal glucose medium lacking methionine for 18 h before the shift to 37°C. mRNA half-lives (t1/2, indicated in minutes) are shown below each panel. (B) Strains were pregrown at 23°C (23°C lanes), shifted to 42°C for 15 min (42°C lanes), and then transferred to 37°C (37°C lanes) for the times indicated. The GAL::lsm8 and nup145N/GAL::lsm8 strains (lanes 37 to 48) were pregrown on galactose and transferred to glucose for 18 h before the heat shock. RNA was separated on 2% agarose gels and hybridized with oligonucleotide probes complementary for the mRNAs indicated on the right. The level of 18S rRNA (probe 008) was used as a loading control. (C and D) Graphic representation of mRNA levels during transcriptional shutdown for MET3 (C) and SSA4 (D) and mRNAs. Values for each RNA were standardized relative to those for the loading control (18S rRNA). RNA levels were calculated based on PhosphorImager quantification of Northern hybridization data from panels A and B. WT, wild type.
FIG. 2.
Heat shock mRNAs stabilized in lsm mutants are polyadenylated. Strains were grown, and total RNA was prepared as described for Fig. 3. (A and B) Strains were pregrown at 23°C, shifted to 42°C for 15 min (42°C lanes), and then transferred to 37°C for 15 or 90 min (37°C lanes). (C) Strains pregrown in minimal medium lacking methionine at 23°C were shifted to 37°C for 30 min (0-min lane), supplemented with methionine to a 2 mM final concentration, and further incubated for 5 or 10 min (5- and 10-min lanes). GAL::lsm8 strains were pregrown on galactose and transferred to glucose medium for 18 h before a temperature shift. RNA samples were annealed with oligonucleotide complementary to either SSA4 (A, oligonucleotide 477), SSA1 (B, oligonucleotide 475), heat shock mRNAs, or MET3 (C, oligonucleotide 486) and treated with RNase H. RNA from the wild type (WT) was also treated with oligo(dT) (lane marked +dT) to visualize deadenylated species. Samples were separated on a 6% acrylamide gel and hybridized with anti-SSA4-3′ probe (A, oligonucleotide 478), anti-SSA1-3′ probe (B, oligonucleotide 476), or anti-MET3-3′ probe (C, oligonucleotide 485). The positions of migration of scR1 (676 nucleotides) and U2 snRNA (1,175 nucleotides) determined by hybridization of the same filters are indicated on the left as size markers.
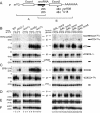
FIG. 3.
5′-unprocessed fragments of pre-mRNAs containing intronic snoRNAs accumulate in lsm mutants. (A) Schematic representation of degradation intermediates for a pre-mRNA containing an intronic snoRNA. The locations of numbered oligonucleotide probes are shown below. P, full-length precursor; A, product of 3′→5′ degradation that extends from the 5′ end of the transcript to the end of the snoRNA; B, product of 5′→3′ degradation that extends from the 5′ end of the snoRNA to the 3′ end of the transcript (see Fig. 3 in reference 11). (B to F) Analysis of pre-mRNA degradation in GAL::lsm and lsm-Δ strains. Strains carrying GAL-regulated constructs (GAL::lsm) (lanes 14 to 28) were grown in permissive RSG medium (0 h) and transferred to repressive, glucose medium at 30°C for the times indicated. Strains in which Lsm1p, Lsm6p, or Lsm7p was deleted (lanes 5 to 13), the temperature-sensitive _prp2_-1 strain (lanes 3 to 4) and the wild-type (WT) strain (lanes 1 to 2) were pregrown at 23°C (0 h) and transferred to 37°C for the times indicated. RNA was separated on 2% agarose gels or on 6% polyacrylamide gel (BIV and CIII) and hybridized with the oligonucleotide probes shown in parentheses. The names of RNA species are on the left or between two columns (BIV and CIII); the positions of pre-mRNAs (P), degradation intermediates (A, A′, and B), and mature mRNAs (M) are indicated, and schematic representations are shown on the right. The level of scR1 RNA is shown as a control for loading.
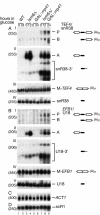
FIG. 4.
Analysis of pre-mRNA degradation in GAL::lsm8, GAL::prp45, lsm2(Ts), and lsm5(Ts) strains. Strains were grown, and RNA was prepared as described for Fig. 3. RNA was separated on 2% agarose gels or on 6% polyacrylamide gel (AIV) and hybridized with the oligonucleotide probes shown in parentheses. The names of RNA species are on the left or between two columns (AIII, AIV); the positions of pre-mRNAs (P), degradation intermediates (A and B), and mature mRNAs (M) are indicated, and schematic representations are shown on the right.
FIG. 5.
Alterations in U6 abundance are not responsible for the defects in nuclear pre-mRNA degradation. RNAs were extracted from strain BSY557 carrying the U6 gene under a promoter containing a LacI binding site and a LacI gene under the GAL1 promoter (BSY557+U6-lacIbs+GAL-lacI; lanes 5 to 8), from its isogenic wild-type (WT) strain (BSY557, lanes 1 to 2) and from the lsm6-Δ strain (lanes 2 to 3) grown in minimal medium with 2% lactate, 2% glycerol, and 0.05% glucose (0 h) and supplemented with 2% galactose. The lsm6Δ strain (lanes 10 to 11), the GAL-lsm8 strain (lanes 14 to 15), BMA64+(pU6 (lane 9), the lsm6Δ+pU6 strain (lanes 12 to 13), and the GAL-lsm8+pU6 strain (lanes 16 to 17) expressing U6 from a high-copy-number 2μm plasmid were grown as described for Fig. 3. RNA was separated on 2% agarose gel (A to C) or on 6% polyacrylamide gel (D and E). The names of RNA species are on the left, and schematic representations of the 5′-unprocessed degradation intermediate (A) and mature mRNA (M) are shown on the right.
FIG. 6.
Inactivation of the nuclear 3′→5′ degradation pathway in lsm6Δ strains. Northern blot analysis of the lsm6-Δ strain deficient in exosome-mediated degradation. Strains were pregrown in galactose medium at 23°C (0 h) and transferred to glucose medium for the times indicated. RNA was separated on 2% agarose gels or on 6% polyacrylamide gel (AIV, BV) and hybridized with the oligonucleotide probes shown in parentheses. The names of RNA species and the positions of pre-mRNAs (P), degradation intermediates (A and B), and 3′-extended snoRNAs are indicated, and schematic representations are shown on the right. WT, wild type.
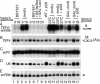
FIG. 7.
mRNAs stabilized in lsm mutants are 5′ capped. (A) Immunoprecipitation of total RNAs with monoclonal anti-cap antibody (H20). RNAs were extracted from the wild type (WT, lanes 1 to 5), nup145N/lsm6-Δ (lanes 6 to 11), and _xrn1-Δ/rat1_-1 (lanes 18 to 23) strains pregrown in glucose at 23°C, shifted to 42°C for 15 min (42°C lanes), and then transferred to 37°C for 90 min (37°C lanes) or from the nup145N/GAL::lsm8 strain pregrown at the permissive RSG medium and transferred to the repressive glucose medium for 18 h prior to the heat shock and shift to 37°C for 90 min. RNAs were immunoprecipitated with H20 or R1131 antibodies or mock treated (lanes marked −Ab) as outlined in Materials and Methods, recovered from the lysate (T), supernatant (S), and immunoprecipitate (P), separated on 2% agarose gels, and analyzed by Northern hybridization using probes complementary to the RNA species indicated on the right. (B) Immunoprecipitation of total RNAs with TMa cap-specific antibody (R1131) or monoclonal antibody against both m7G and m3G cap structures (H20). RNAs were extracted from the wild-type strain BMA64 (WT, lanes 1 to 5) and the lsm6Δ strain (lanes 9 to 15) grown on glucose at 23°C, from the _prp2_-1 strain (lanes 6 to 8) pregrown at 23°C and transferred to 37°C for 3 h, and from the GAL::lsm8 strain pregrown in permissive RSG medium and transferred to the repressive glucose medium for 18 h.
FIG. 8.
Lsm8p can be cross-linked to polyadenylated nuclear RNAs. A Western blot of proteins UV cross-linked to poly(A)+ RNAs is shown. The Lsm8-TAP strain was grown at 30°C to an OD600 of 0.75, and the nup145N/Lsm8-TAP strain was pregrown at 23°C to an OD600 of 0.6 and transferred to 37°C for 30 min to block mRNA export. UV cross-linking and purification of poly(A)+ RNPs on oligo(dT)-cellulose was performed as described in Materials and Methods. Western analysis was performed on total lysate (T) and on RNP complexes recovered from the nonirradiated (−) and irradiated (+) cells resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis following RNase digestion of the bound material. Approximately 90-fold more material was loaded for the eluate fractions compared to the total fractions. Western blot analysis was decorated with anti-protein A Ab to detect ProtA-Nup145N (A), Lsm8-TAP (C), anti-Npl3 Ab (B), and anti-Nop1p Ab (D).
Similar articles
- Requirements for nuclear localization of the Lsm2-8p complex and competition between nuclear and cytoplasmic Lsm complexes.
Spiller MP, Reijns MA, Beggs JD. Spiller MP, et al. J Cell Sci. 2007 Dec 15;120(Pt 24):4310-20. doi: 10.1242/jcs.019943. Epub 2007 Nov 20. J Cell Sci. 2007. PMID: 18029398 Free PMC article. - A Sm-like protein complex that participates in mRNA degradation.
Bouveret E, Rigaut G, Shevchenko A, Wilm M, Séraphin B. Bouveret E, et al. EMBO J. 2000 Apr 3;19(7):1661-71. doi: 10.1093/emboj/19.7.1661. EMBO J. 2000. PMID: 10747033 Free PMC article. - Yeast Sm-like proteins function in mRNA decapping and decay.
Tharun S, He W, Mayes AE, Lennertz P, Beggs JD, Parker R. Tharun S, et al. Nature. 2000 Mar 30;404(6777):515-8. doi: 10.1038/35006676. Nature. 2000. PMID: 10761922 - Mechanisms and control of mRNA decapping in Saccharomyces cerevisiae.
Tucker M, Parker R. Tucker M, et al. Annu Rev Biochem. 2000;69:571-95. doi: 10.1146/annurev.biochem.69.1.571. Annu Rev Biochem. 2000. PMID: 10966469 Review. - 3' Uridylation and the regulation of RNA function in the cytoplasm.
Norbury CJ. Norbury CJ. Biochem Soc Trans. 2010 Aug;38(4):1150-3. doi: 10.1042/BST0381150. Biochem Soc Trans. 2010. PMID: 20659020 Review.
Cited by
- RNA Metabolism and the Role of Small RNAs in Regulating Multiple Aspects of RNA Metabolism.
Dawar P, Adhikari I, Mandal SN, Jayee B. Dawar P, et al. Noncoding RNA. 2024 Dec 24;11(1):1. doi: 10.3390/ncrna11010001. Noncoding RNA. 2024. PMID: 39846679 Free PMC article. Review. - RNA modifications and Prp24 coordinate Lsm2-8 binding dynamics during S. cerevisiae U6 snRNP assembly.
Liu Y, Nomura Y, Butcher SE, Hoskins AA. Liu Y, et al. J Biol Chem. 2025 May;301(5):108497. doi: 10.1016/j.jbc.2025.108497. Epub 2025 Apr 10. J Biol Chem. 2025. PMID: 40216252 Free PMC article. - Arabidopsis thaliana LSM proteins function in mRNA splicing and degradation.
Golisz A, Sikorski PJ, Kruszka K, Kufel J. Golisz A, et al. Nucleic Acids Res. 2013 Jul;41(12):6232-49. doi: 10.1093/nar/gkt296. Epub 2013 Apr 24. Nucleic Acids Res. 2013. PMID: 23620288 Free PMC article. - Kiss your tail goodbye: the role of PARN, Nocturnin, and Angel deadenylases in mRNA biology.
Godwin AR, Kojima S, Green CB, Wilusz J. Godwin AR, et al. Biochim Biophys Acta. 2013 Jun-Jul;1829(6-7):571-9. doi: 10.1016/j.bbagrm.2012.12.004. Epub 2012 Dec 26. Biochim Biophys Acta. 2013. PMID: 23274303 Free PMC article. Review. - RNA-binding proteins with prion-like domains in health and disease.
Harrison AF, Shorter J. Harrison AF, et al. Biochem J. 2017 Apr 7;474(8):1417-1438. doi: 10.1042/BCJ20160499. Biochem J. 2017. PMID: 28389532 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous