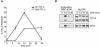Lipopolysaccharide activation of the TPL-2/MEK/extracellular signal-regulated kinase mitogen-activated protein kinase cascade is regulated by IkappaB kinase-induced proteolysis of NF-kappaB1 p105 - PubMed (original) (raw)
Lipopolysaccharide activation of the TPL-2/MEK/extracellular signal-regulated kinase mitogen-activated protein kinase cascade is regulated by IkappaB kinase-induced proteolysis of NF-kappaB1 p105
S Beinke et al. Mol Cell Biol. 2004 Nov.
Abstract
The MEK kinase TPL-2 (also known as Cot) is required for lipopolysaccharide (LPS) activation of the extracellular signal-regulated kinase (ERK) mitogen-activated protein (MAP) kinase cascade in macrophages and consequent upregulation of genes involved in innate immune responses. In resting cells, TPL-2 forms a stoichiometric complex with NF-kappaB1 p105, which negatively regulates its MEK kinase activity. Here, it is shown that lipopolysaccharide (LPS) stimulation of primary macrophages causes the release of both long and short forms of TPL-2 from p105 and that TPL-2 MEK kinase activity is restricted to this p105-free pool. Activation of TPL-2, MEK, and ERK by LPS is also demonstrated to require proteasome-mediated proteolysis. p105 is known to be proteolysed by the proteasome following stimulus-induced phosphorylation of two serines in its PEST region by the IkappaB kinase (IKK) complex. Expression of a p105 point mutant, which is not susceptible to signal-induced proteolysis, in RAW264.7 macrophages impairs LPS-induced release of TPL-2 from p105 and its subsequent activation of MEK. Furthermore, expression of wild-type but not mutant p105 reconstitutes LPS stimulation of MEK and ERK phosphorylation in primary NF-kappaB1-deficient macrophages. Consistently, pharmacological blockade of IKK inhibits LPS-induced release of TPL-2 from p105 and TPL-2 activation. These data show that IKK-induced p105 proteolysis is essential for LPS activation of TPL-2, thus revealing a novel function of IKK in the regulation of the ERK MAP kinase cascade.
Figures
FIG. 1.
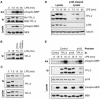
LPS stimulation induces a p105-free pool of TPL-2 which activates MEK. BMDMs (BALB/c) were stimulated with LPS for the indicated times. (A) TPL-2 was immunoprecipitated from cell lysates with anti-TPL-2 antibody, and its MEK kinase activity was determined by coupled MEK/ERK kinase assay. Labeled MBP substrate was visualized by autoradiography after SDS-PAGE, and the levels of immunoprecipitated TPL-2 were determined by Western blotting. Lysates were also subjected to Western blotting with an anti-phospho-(S217/S221)-MEK-1/2 antibody (phospho-MEK) to determine activation of endogenous MEK by LPS. (B) TPL-2 was immunoprecipitated from cell lysates. Beads were incubated with 0.5 U of PP2A, 0.5 U of PP2A plus the phosphatase inhibitors NaF and okadaic acid (PP2A + Inhibitors), or control buffer. Immunoprecipitated TPL-2 was revealed by Western blotting. (C) Cell lysates and anti-p105 immunoprecipitates were subjected to Western blotting. (D) Total cell lysates (lysates) and p105 cleared lysates were subjected to Western blotting for TPL-2 and p105. Equal protein loading was confirmed by probing for α-tubulin. (E) Cell lysates were immunoprecipitated with anti-TPL-2 antibody, anti-p105N antibody, or control Ig (control) after first immunodepleting with either anti-p105N antibody (p105) or control Ig (control) as indicated. Immunoprecipitates were assayed for associated MEK kinase activity and TPL-2 levels as for panel A. Cell lysates were subjected to Western blotting to confirm depletion of p105 and activation of MEK phosphorylation by LPS.
FIG. 2.
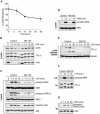
Proteasome activity is required for LPS activation of TPL-2. (A) BMDMs were simulated with LPS for the indicated times. Total p105 protein levels in cell lysates were determined by Western blotting and quantified using a Fuji Image reader. Data are presented as means (± SEM; n = 3), normalized against α-tubulin. (B to E) BMDMs were preincubated with the proteasome inhibitor MG132 (40 μM) or DMSO vehicle control for 30 min and then stimulated with LPS or PMA for the indicated times. Total cell lysates were subjected to Western blotting for the indicated proteins. LPS activation of endogenous MEK, ERK, and p38 was monitored with the appropriate phospho-specific antibodies. (E) Cell lysates were immunodepleted of p105 prior to Western blotting for TPL-2 and α-tubulin. (F) BMDMs were treated with MG132 (40 μM) prior to stimulation with LPS for 15 min. TPL-2 was immunoprecipitated from total cell lysates and then assayed for MEK kinase activity as for Fig. 1A. (G) BMDMs, pretreated with 40 μM MG132, were simulated with LPS for the indicated times. p105 was immunoprecipitated from total cell lysates, subjected to Western blotting, and probed with a specific anti-phospho-peptide antibody to monitor p105 serine 927 phosphorylation.
FIG. 3.
LPS activation of MEK in RAW264.7 cells is dependent on TPL-2 and proteasome activity. (A) RAW 264.7 cells stably transfected with expression vectors encoding wild-type Myc-TPL-2 (WT), kinase-inactive Myc-TPL-2 (KD), or no insert control (empty vector [EV]) were stimulated with LPS, and cell lysates were subjected to Western blotting. LPS activation of endogenous MEK and p38 activation were assayed using phospho-specific antibodies. (B to D) RAW264.7 cells were preincubated with MG132 (40 μM) or DMSO vehicle control for 30 min and then stimulated with LPS for the times indicated. (B) Total cell lysates were subjected to Western blotting for the indicated proteins. LPS activation of MEK and p38 activation were monitored with the phospho-specific antibodies. (C) Cell lysates were immunodepleted of p105 and Western blotted for TPL-2 and α-tubulin. (D) TPL-2 was immunoprecipitated from total cell lysates and then assayed for MEK kinase activity as for Fig. 1A.
FIG. 4.
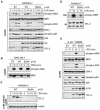
LPS activation of TPL-2 is dependent on signal-induced p105 proteolysis. (A to D) RAW 264.7 cells stably transfected with vectors encoding wild-type HA-p105 (WT), HA-p105S927A,S932A (SSAA), or with no insert (EV) were stimulated with LPS or PMA for the indicated times. (A and B) Total cell lysates were subjected to Western blotting, and phospho-specific antibodies were used to monitor MEK, ERK, and p38 activation. (C) Lysates were immunodepleted of p105 and subjected to Western blotting for TPL-2 and α-tubulin. (D) TPL-2 was immunoprecipitated from lysates of RAW264.7 cells expressing HA-p105 (WT) or HA-p105S927A,S932A (SSAA), and MEK kinase activity was determined by a coupled MEK/ERK kinase assay as for Fig. 1A. (E) BMDMs generated from NF-κB1−/− mice were infected with recombinant retroviruses encoding wild-type FL-p105 (WT), FL-p105S927A,S932A (SSAA), or with no insert (EV). Cells were stimulated with LPS (10 ng/ml) for 15 min or left untreated, and total cell lysates were subjected to Western blotting for the indicated proteins. Endogenous MEK, ERK, and p38 activation was assayed using phospho-specific antibodies.
FIG. 5.
IKK is required for LPS activation of TPL-2. BMDMs (BALB/c) were preincubated with the IKK inhibitor BAY11-7082 or DMSO vehicle control and stimulated with LPS or PMA for the indicted times. BAY11-7082 was used at a concentration of 7.5 μM unless otherwise indicated. (A to C) Total cell lysates were subjected to Western blotting for the indicated proteins. (D) Lysates were immunodepleted of p105 and subjected to Western blotting for TPL-2 and α-tubulin. (E) TPL-2 was immunoprecipitated from total cell lysates and assayed for MEK kinase activity in a coupled MEK/ERK kinase assay as for Fig. 1A.
FIG. 6.
IKK preferentially phosphorylates p105 complexed with M1-TPL-2. BMDMs (BALB/c) were simulated with LPS for the indicated times. (A) Total cell lysates or cell lysates depleted of p105 were subjected to Western blotting for TPL-2. Bands were quantified by densitometry, and data are presented as a mean (± SEM; n = 4) of the fraction of M1- or M30-TPL-2 that is released from p105 normalized against α-tubulin levels. (B) Total p105 or p105 phosphorylated on S927 was immunoprecipitated from total cell lysates by using anti-p105N or anti-phospho-S927-p105 antibodies, respectively. Associated TPL-2 was detected by Western blotting after SDS-PAGE.
Similar articles
- Phosphorylation of TPL-2 on serine 400 is essential for lipopolysaccharide activation of extracellular signal-regulated kinase in macrophages.
Robinson MJ, Beinke S, Kouroumalis A, Tsichlis PN, Ley SC. Robinson MJ, et al. Mol Cell Biol. 2007 Nov;27(21):7355-64. doi: 10.1128/MCB.00301-07. Epub 2007 Aug 20. Mol Cell Biol. 2007. PMID: 17709378 Free PMC article. - Coordinate regulation of TPL-2 and NF-κB signaling in macrophages by NF-κB1 p105.
Yang HT, Papoutsopoulou S, Belich M, Brender C, Janzen J, Gantke T, Handley M, Ley SC. Yang HT, et al. Mol Cell Biol. 2012 Sep;32(17):3438-51. doi: 10.1128/MCB.00564-12. Epub 2012 Jun 25. Mol Cell Biol. 2012. PMID: 22733995 Free PMC article. - IκB kinase 2 regulates TPL-2 activation of extracellular signal-regulated kinases 1 and 2 by direct phosphorylation of TPL-2 serine 400.
Roget K, Ben-Addi A, Mambole-Dema A, Gantke T, Yang HT, Janzen J, Morrice N, Abbott D, Ley SC. Roget K, et al. Mol Cell Biol. 2012 Nov;32(22):4684-90. doi: 10.1128/MCB.01065-12. Epub 2012 Sep 17. Mol Cell Biol. 2012. PMID: 22988300 Free PMC article. - Regulation and function of TPL-2, an IκB kinase-regulated MAP kinase kinase kinase.
Gantke T, Sriskantharajah S, Ley SC. Gantke T, et al. Cell Res. 2011 Jan;21(1):131-45. doi: 10.1038/cr.2010.173. Epub 2010 Dec 7. Cell Res. 2011. PMID: 21135874 Free PMC article. Review. - IκB kinase regulation of the TPL-2/ERK MAPK pathway.
Gantke T, Sriskantharajah S, Sadowski M, Ley SC. Gantke T, et al. Immunol Rev. 2012 Mar;246(1):168-82. doi: 10.1111/j.1600-065X.2012.01104.x. Immunol Rev. 2012. PMID: 22435554 Review.
Cited by
- The interplay between cyclic AMP, MAPK, and NF-κB pathways in response to proinflammatory signals in microglia.
Ghosh M, Aguirre V, Wai K, Felfly H, Dietrich WD, Pearse DD. Ghosh M, et al. Biomed Res Int. 2015;2015:308461. doi: 10.1155/2015/308461. Epub 2015 Feb 5. Biomed Res Int. 2015. PMID: 25722974 Free PMC article. - Activation Receptor-Dependent IFN-γ Production by NK Cells Is Controlled by Transcription, Translation, and the Proteasome.
Piersma SJ, Pak-Wittel MA, Lin A, Plougastel-Douglas B, Yokoyama WM. Piersma SJ, et al. J Immunol. 2019 Oct 1;203(7):1981-1988. doi: 10.4049/jimmunol.1900718. Epub 2019 Aug 23. J Immunol. 2019. PMID: 31444264 Free PMC article. - Tpl2 kinase is upregulated in adipose tissue in obesity and may mediate interleukin-1beta and tumor necrosis factor-{alpha} effects on extracellular signal-regulated kinase activation and lipolysis.
Jager J, Grémeaux T, Gonzalez T, Bonnafous S, Debard C, Laville M, Vidal H, Tran A, Gual P, Le Marchand-Brustel Y, Cormont M, Tanti JF. Jager J, et al. Diabetes. 2010 Jan;59(1):61-70. doi: 10.2337/db09-0470. Epub 2009 Oct 6. Diabetes. 2010. PMID: 19808894 Free PMC article. - A single NFκB system for both canonical and non-canonical signaling.
Shih VF, Tsui R, Caldwell A, Hoffmann A. Shih VF, et al. Cell Res. 2011 Jan;21(1):86-102. doi: 10.1038/cr.2010.161. Epub 2010 Nov 23. Cell Res. 2011. PMID: 21102550 Free PMC article. Review. - Distinct roles for IκB kinases alpha and beta in regulating pulmonary endothelial angiogenic function during late lung development.
Iosef C, Liu M, Ying L, Rao SP, Concepcion KR, Chan WK, Oman A, Alvira CM. Iosef C, et al. J Cell Mol Med. 2018 Sep;22(9):4410-4422. doi: 10.1111/jcmm.13741. Epub 2018 Jul 11. J Cell Mol Med. 2018. PMID: 29993183 Free PMC article.
References
- Aoki, M., F. Hamada, T. Sugimoto, S. Sumida, T. Akiyama, and K. Toyoshima. 1993. The human cot proto-oncogene encodes two protein serine/threonine kinases with different transforming activities by alternative initiation of translation. J. Biol. Chem. 268:22723-22732. - PubMed
- Beinke, S., M. P. Belich, and S. C. Ley. 2002. The death domain of NF-κB1 p105 is essential for signal-induced p105 proteolysis. J. Biol. Chem. 277:24162-24168. - PubMed
- Belich, M. P., A. Salmeron, L. H. Johnston, and S. C. Ley. 1999. TPL-2 kinase regulates the proteolysis of the NF-κB inhibitory protein NF-κB1 p105. Nature 397:363-368. - PubMed
- Burke, J. R., M. A. Pattoli, K. R. Gregor, P. J. Brassil, J. F. MacMaster, K. W. McIntyre, X. Yang, V. S. Iotzova, W. Clarke, J. Strnad, Y. Qiu, and F. C. Zusi. 2003. BMS-345541 is a highly selective inhibitor of I kappa B kinase that binds at an allosteric site of the enzyme and blocks NF-kappa B-dependent transcription in mice. J. Biol. Chem. 278:1450-1456. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous