Distinct molecular forms of beta-catenin are targeted to adhesive or transcriptional complexes - PubMed (original) (raw)
Distinct molecular forms of beta-catenin are targeted to adhesive or transcriptional complexes
Cara J Gottardi et al. J Cell Biol. 2004.
Abstract
Beta-catenin plays essential roles in both cell-cell adhesion and Wnt signal transduction, but what precisely controls beta-catenin targeting to cadherin adhesive complexes, or T-cell factor (TCF)-transcriptional complexes is less well understood. We show that during Wnt signaling, a form of beta-catenin is generated that binds TCF but not the cadherin cytoplasmic domain. The Wnt-stimulated, TCF-selective form is monomeric and is regulated by the COOH terminus of beta-catenin, which selectively competes cadherin binding through an intramolecular fold-back mechanism. Phosphorylation of the cadherin reverses the TCF binding selectivity, suggesting another potential layer of regulation. In contrast, the main cadherin-binding form of beta-catenin is a beta-catenin-alpha-catenin dimer, indicating that there is a distinct molecular form of beta-catenin that can interact with both the cadherin and alpha-catenin. We propose that participation of beta-catenin in adhesion or Wnt signaling is dictated by the regulation of distinct molecular forms of beta-catenin with different binding properties, rather than simple competition between cadherins and TCFs for a single constitutive form. This model explains how cells can control whether beta-catenin is used independently in cell adhesion and nuclear signaling, or competitively so that the two processes are coordinated and interrelated.
Figures
Figure 1.
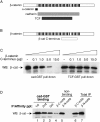
Wnt signaling generates a form of β-catenin that binds preferentially to TCF-GST compared with cadherin-GST. (A) Detergent-free supernatants were prepared from C57MG and Rat1 cells stably expressing Wnt-1, and HEK293T cells incubated overnight ± Wnt3a-conditioned media (CM). Samples were affinity precipitated using equimolar amounts of cad-GST or TCF-GST fusion proteins. GST gives no binding and is not depicted. A fivefold excess of parental cell lysates was required to detect a signal in lanes 5 and 6. Cytosolic β-catenin from C57MG parentals binds cad-GST and TCF-GST proteins equivalently, like the Rat1 and HEK293 controls (not depicted). The blot was probed with a pAb to β-catenin. (B) Preferential binding of β-catenin to TCF-GST over cadherin-GST is not observed with purified, recombinant β-catenin. Recombinant, purified Xenopus β-catenin (Suh and Gumbiner, 2003) and β-catenin from a C57MG/Wnt cytosolic fraction were affinity precipitated with cad-GST and TCF-GST proteins, and blotted with an antibody to β-catenin.
Figure 2.
The COOH terminus of β-catenin restricts binding to cadherin. COOH terminus of β-catenin competes cadherin, but not TCF binding. (A) Schematic shows where α-catenin, cadherin, and TCF interact with β-catenin (Huber et al., 1997; Graham et al., 2000; Pokutta and Weis, 2000; Huber and Weis, 2001). (B) The COOH terminus of β-catenin binds the arm repeat region of β-catenin in yeast-two hybrid (Cox et al., 1999) and recombinant protein assays (Piedra et al., 2001). (C) COOH-terminal region of β-catenin competes β-catenin binding to cad-GST, but not to TCF-GST fusion protein_._ Recombinant β-catenin (1.5 μg) purified from baculovirus (Suh and Gumbiner, 2003) was incubated with cadherin-GST (2 μg) or TCF-GST (2.4 μg) coupled agarose beads in the presence of increasing amounts of β-catenin COOH-terminal peptide (amino acids 695–781). Affinity precipitates were analyzed by SDS-PAGE and Western blotting with an antibody to β-catenin. (D) Cadherin-GST preferentially depletes the fraction of β-catenin recognized by a COOH-terminal mAb (M5.2). A cytosolic fraction from Rat1/Wnt cells was affinity precipitated (×3) with cadherin-GST (lanes 1–3). The cad-GST nonbinding pool (lanes 4 and 5) was divided in two and immunoprecipitated with either an mAb that recognizes a COOH-terminal β-catenin epitope (βC-mAb (M5.2), lane 4) or an NH2-terminal β-catenin epitope (βN-mAb (1.1), lane 5). As a control, these antibodies were used to immunoprecipitate β-catenin from the total starting material (not previously depleted with cad-GST; lanes 6 and 7).
Figure 3.
Differential binding activity of recombinant β-catenin as revealed by deletion analysis. (A) Schematic representation of β-catenin constructs. WT-myc-Xenopus β-catenin and GSK3β mutant (S/T>A residues 33, 37, 41, and 45) β-catenin were described previously by Guger and Gumbiner (2000). WT-human β-catenin-flag, ΔC695-flag and ΔN89-flag constructs were described in Kolligs et al. (1999). The myc-tagged, Xenopus β-catenin construct encoding only the arm repeat region of β-catenin was described previously (Funayama et al., 1995). (B) Recombinant β-catenin binding to cad-GST versus TCF-GST proteins. HEK293T cells were transfected with decreasing amounts of β-catenin plasmid and incubated in the presence (+) of Wnt3a conditioned media (CM). Cytosolic fractions were affinity precipitated and immunoblotted with anti-myc, -flag, or β-catenin antibodies. Input amounts of wild-type β-catenin, −ΔC695, and arm 12 constructs were the same in accordance with similar expression levels (not depicted).
Figure 4.
Larger molecular size, α-catenin–containing fractions of β-catenin show preferential binding to cad-GST. (A) A cytosolic fraction from stage 12 Xenopus embryos was applied to a Sephacryl 300 gel filtration column, and fractions 28–39 were divided in two: one half of each sample was TCA-precipitated (top blot), whereas the other half was precipitated with cad-GST (middle blot). The top blot was reprobed with an antibody to α-catenin and is shown below. (B) Same as A except that starting material is an S100 fraction from Rat1/Wnt cells. Arrows refer to elution volumes of standard proteins with known molecular weight: (a) catalase (Mr = 232,000); (b) BSA (Mr = 66,000), purified mouse IgG (150 kD) eluted in fractions 31–33.
Figure 5.
The α-catenin–free, monomeric form of β-catenin exhibits preferential binding to TCF compared with cadherin in Wnt cells. (A) Rat1 cells were labeled to steady-state with [35S]methionine/cysteine, and a cytosolic fraction was prepared from each condition (−Wnt, +Wnt, 10 mM LiCl, 12 h) and immunoprecipitated with the designated antibodies or affinity precipitated with GST proteins. Note that immunoprecipitation of endogenous E-cadherin (from the 100,000 g membrane pellet, lanes 5, 10, and 16) and TCF (lane 11) are also shown. Non-specific bands were not seen with a GST control (not depicted). Overnight incubation with LiCl (10 mM) allows the α-catenin–free pool of β-catenin to bind cad-GST, TCF-GST, and the endogenous E-cadherin (lanes 14–16), equivalently. (B). COOH-terminal epitopes of β-catenin are masked in the α-catenin–free fraction of β-catenin. Equivalent amounts of an S100 fraction from [35S]methionine/cysteine steady-state–labeled Rat1+Wnt cells were immunoprecipitated with the following antibodies: anti–β-catenin NH2-terminal mAb (1.1.1; lane 1), anti–β-catenin COOH-terminal mAb (M5.2; lane 2), anti–α-catenin mAb (lane 4), and a nonimmune control (lane 3). (Lanes 5–7) PDZ protein, mLin7, preferentially binds to β-catenin–α-catenin dimer: metabolically labeled Rat1+Wnt lysates were affinity precipitated with (lane 5) anti–β-catenin pAb, (lane 6) control GST, and (lane 7) mLin7-GST.
Figure 6.
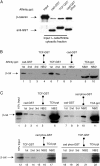
β-Catenin binding selectivity as a function of APC mutant status or GSK inhibition by LiCl. (A) A cytosolic fraction was prepared from colon carcinoma cell lines containing wild-type (HCT116) or mutant (HT29 and DLD1) forms of APC. (B) Selective binding activity of β-catenin in response to short-term, but not long-term treatment with LiCl. HEK293T cells were treated with 10 mM LiCl for 3, 6, and 15 h, after which cytosolic fractions were affinity precipitated as described above.
Figure 7.
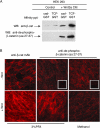
β-Catenin not phosphorylated at NH 2 -terminal GSK-3β sites binds to cadherin. (A) Cytosolic fraction from HEK293 cells ± Wnt3a was affinity precipitated with cad-GST and TCF-GST, and blotted with pAbs to β-catenin (top blot) or NH2-terminal unphosphorylated–β-catenin (amino acids 27–37, bottom blot). (B) NH2-terminal unphospho–β-catenin localizes to sites of cell–cell contact in Wnt-expressing cells. Rat1 fibroblasts ± Wnt were fixed and processed for immunofluorescence using standard protocols. Images were captured with the Axioplan 2 microscope and AxioVision2.0 software (Carl Zeiss MicroImaging, Inc.). Note that membrane staining of the unphospho-β-catenin (Cy3) is more readily detected under methanol, rather than PFA fixation conditions, perhaps accounting for the apparent differences observed between our study and Staal et al. (2002).
Figure 8.
Cadherin phosphorylation reverses β-catenin binding selectivity during Wnt signaling. (A) Phosphorylation of cad-GST increases β-catenin binding to cadherin compared with TCF. A cytosolic fraction from L cells transfected with Wnt3a were incubated with equimolar amounts of cad-GST, TCF-GST, and CK2-P-cad-GST-glutathione–coupled beads for 1 h at 4°C (see Fig. S1 for characterization of GST fusion proteins). The resulting anti–β-catenin and anti-GST immunoblots are shown. (B) Fraction of β-catenin that binds cadherin is a subset of fraction of β-catenin that binds TCF. Cytosolic fraction of Wnt cells was sequentially affinity precipitated with cad-GST (lanes 1–3) or TCF-GST (lanes 6–8) proteins. After cad-GST depletion (lanes 1–3), half of the cad-GST non-binding fraction (NB/2) was precipitated with TCF-GST (lane 4); the other half was precipitated with TCA to show amount remaining (lane 5, far right). After TCF-GST depletion (lanes 6–8), half of the TCF-GST non-binding fraction (NB/2, lane 9) was precipitated with cad-GST, whereas the other half was precipitated with TCA to show amount remaining (lane 10, far right). Lanes 5 and 10 reveal a fraction of β-catenin that binds neither TCF nor cadherin. This fraction is likely due to β-catenin already complexed with partners such as ICAT (Gottardi and Gumbiner, 2004). (C) Phosphorylated cadherin-GST and TCF-GST bind the same pool of β-catenin in Wnt-activated cells. Cytosolic fraction was precipitated with cad-GST (top blot), TCF-GST (bottom left) or P-cadherin-GST (bottom right) fusion proteins. After cad-GST depletion (lanes 2–4 and 7–9), there is a fraction of β-catenin that binds TCF-GST (lane 5) and P-cadherin-GST (lane 10). Note that after TCF-GST depletion (lanes 13–15), there is no β-catenin remaining to bind P-cadherin-GST (lane 16). After P-cadherin-GST depletion (lanes 18–20), there is no β-catenin remaining to bind TCF-GST (lane 21). Reciprocal depletions suggest that P-cadherin-GST and TCF-GST bind the same form of β-catenin.
Figure 9.
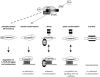
Multiple forms of β-catenin exist in cells. NH2-terminal phospho-β-catenin is well characterized and generated by the APC-Axin-GSK3β-CK1 complex (dashed line). Closed form of β-catenin is generated by Wnt signaling, perhaps through some of the same machinery (gray arrow). β-Catenin–α-catenin dimer is active for adhesion but not signaling. Open form binds both cadherin and TCF, and could explain how cadherin antagonizes β-catenin signaling in overexpression systems. The inactive form cannot participate in adhesion or signaling.
Similar articles
- E-cadherin suppresses cellular transformation by inhibiting beta-catenin signaling in an adhesion-independent manner.
Gottardi CJ, Wong E, Gumbiner BM. Gottardi CJ, et al. J Cell Biol. 2001 May 28;153(5):1049-60. doi: 10.1083/jcb.153.5.1049. J Cell Biol. 2001. PMID: 11381089 Free PMC article. - Role for ICAT in beta-catenin-dependent nuclear signaling and cadherin functions.
Gottardi CJ, Gumbiner BM. Gottardi CJ, et al. Am J Physiol Cell Physiol. 2004 Apr;286(4):C747-56. doi: 10.1152/ajpcell.00433.2003. Epub 2003 Nov 12. Am J Physiol Cell Physiol. 2004. PMID: 14613891 - Requirement for a nuclear function of beta-catenin in Wnt signaling.
Cong F, Schweizer L, Chamorro M, Varmus H. Cong F, et al. Mol Cell Biol. 2003 Dec;23(23):8462-70. doi: 10.1128/MCB.23.23.8462-8470.2003. Mol Cell Biol. 2003. PMID: 14612392 Free PMC article. - The Yin-Yang of TCF/beta-catenin signaling.
Barker N, Morin PJ, Clevers H. Barker N, et al. Adv Cancer Res. 2000;77:1-24. doi: 10.1016/s0065-230x(08)60783-6. Adv Cancer Res. 2000. PMID: 10549354 Review. - TCF: Lady Justice casting the final verdict on the outcome of Wnt signalling.
Brantjes H, Barker N, van Es J, Clevers H. Brantjes H, et al. Biol Chem. 2002 Feb;383(2):255-61. doi: 10.1515/BC.2002.027. Biol Chem. 2002. PMID: 11934263 Review.
Cited by
- Allele-specific endogenous tagging and quantitative analysis of β-catenin in colorectal cancer cells.
Ambrosi G, Voloshanenko O, Eckert AF, Kranz D, Nienhaus GU, Boutros M. Ambrosi G, et al. Elife. 2022 Jan 11;11:e64498. doi: 10.7554/eLife.64498. Elife. 2022. PMID: 35014953 Free PMC article. - Hydrocephalus and abnormal subcommissural organ in mice lacking presenilin-1 in Wnt1 cell lineages.
Nakajima M, Matsuda K, Miyauchi N, Fukunaga Y, Watanabe S, Okuyama S, Pérez J, Fernández-Llebrez P, Shen J, Furukawa Y. Nakajima M, et al. Brain Res. 2011 Mar 25;1382:275-81. doi: 10.1016/j.brainres.2011.01.048. Epub 2011 Jan 22. Brain Res. 2011. PMID: 21262207 Free PMC article. - Independent interactions of phosphorylated β-catenin with E-cadherin at cell-cell contacts and APC at cell protrusions.
Faux MC, Coates JL, Kershaw NJ, Layton MJ, Burgess AW. Faux MC, et al. PLoS One. 2010 Nov 30;5(11):e14127. doi: 10.1371/journal.pone.0014127. PLoS One. 2010. PMID: 21152425 Free PMC article. - Pathology and pathogenesis of craniopharyngiomas.
Larkin SJ, Ansorge O. Larkin SJ, et al. Pituitary. 2013 Mar;16(1):9-17. doi: 10.1007/s11102-012-0418-4. Pituitary. 2013. PMID: 22886701 Review. - MT2-MMP induces proteolysis and leads to EMT in carcinomas.
Liu Y, Sun X, Feng J, Deng LL, Liu Y, Li B, Zhu M, Lu C, Zhou L. Liu Y, et al. Oncotarget. 2016 Jul 26;7(30):48193-48205. doi: 10.18632/oncotarget.10194. Oncotarget. 2016. PMID: 27374080 Free PMC article.
References
- Caca, K., F.T. Kolligs, X. Ji, M. Hayes, J. Qian, A. Yahanda, D.L. Rimm, J. Costa, and E.R. Fearon. 1999. Beta- and gamma-catenin mutations, but not E-cadherin inactivation, underlie T-cell factor/lymphoid enhancer factor transcriptional deregulation in gastric and pancreatic cancer. Cell Growth Differ. 10:369–376. - PubMed
- Castano, J., I. Raurell, J.A. Piedra, S. Miravet, M. Dunach, and A. Garcia de Herreros. 2002. β-catenin N- and C-terminal tails modulate the coordinated binding of adherens junction proteins to β-catenin. J. Biol. Chem. 277:31541–31550. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous