CTLA-4-Ig activates forkhead transcription factors and protects dendritic cells from oxidative stress in nonobese diabetic mice - PubMed (original) (raw)
CTLA-4-Ig activates forkhead transcription factors and protects dendritic cells from oxidative stress in nonobese diabetic mice
Francesca Fallarino et al. J Exp Med. 2004.
Abstract
Prediabetes and diabetes in nonobese diabetic (NOD) mice have been targeted by a variety of immunotherapies, including the use of a soluble form of cytotoxic T lymphocyte antigen 4 (CTLA-4) and interferon (IFN)-gamma. The cytokine, however, fails to activate tolerogenic properties in dendritic cells (DCs) from highly susceptible female mice early in prediabetes. The defect is characterized by impaired induction of immunosuppressive tryptophan catabolism, is related to transient blockade of the signal transducer and activator of transcription (STAT)1 pathway of intracellular signaling by IFN-gamma, and is caused by peroxynitrite production. Here, we show that soluble CTLA-4 imparts suppressive properties to DCs from early prediabetic NOD female mice through mechanisms that rely on autocrine signaling by IFN-gamma. Although phosphorylation of STAT1 in response to IFN-gamma is compromised in those mice, CTLA-4 obviates the defect. IFN-gamma-driven expression of tryptophan catabolism by CTLA-4-immunoglobulin is made possible through the concomitant activation of the Forkhead Box class O (FOXO) transcription factor FOXO3a, induction of the superoxide dismutase gene, and prevention of peroxynitrite formation.
Figures
Figure 1.
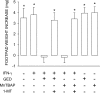
Ability of the SOD mimetic MnTBAP to activate IDO-dependent tolerizing properties in CD8+ DCs from early prediabetic NOD female mice. Combinations of IL-12–treated CD8− and CD8+ DCs subjected to various treatments (indicated) were loaded with NRP-A7 and injected into recipient mice to be assayed at 2 wk for skin test reactivity to the eliciting peptide. CD8− DCs were used after overnight activation with 100 ng/ml IL-12, whereas CD8+ DCs were used either as such or after activation with 200 U/ml IFN-γ in the presence or absence of 250 μM GED or 100 μM MnTBAP. Groups of CD8+ DCs were also treated with 2 μM 1-MT. *, P < 0.001, experimental versus control footpads. One experiment is reported representative of three.
Figure 2.
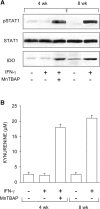
Ability of MnTBAP to restore IFN-γ responsiveness in CD8+ DCs from early prediabetic NOD female mice. (A) MnTBAP was examined for ability to restore STAT1 phosphorylation and IDO expression in response to IFN-γ. CD8+ DCs from 4-wk-old or control 8-wk-old NOD female mice were treated with IFN-γ as specified in Materials and Methods and assayed by Western blot. IDO expression was investigated with an IDO-specific polyclonal antibody, whereas STAT1 phosphorylation was analyzed with an anti-phosphoSTAT1 reagent. One experiment representative of three is shown. (B) MnTBAP was also studied for its ability to restore tryptophan conversion to kynurenine in response to IFN-γ, using CD8+ DCs from the same donors. Kynurenine levels in supernatants were measured by HPLC, and results are the mean ± SD of triplicate samples in one of three experiments.
Figure 3.
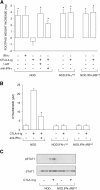
Ability of CTLA-4–Ig to rescue the tolerizing properties of CD8+ DCs from early prediabetic NOD female mice through mechanisms dependent on IFN-γ and IDO. (A) Mixtures of CD8− and CD8+ DCs were loaded with NRP-A7 and injected into recipient mice that were later assayed for skin test reactivity to the peptide. CD8− DCs were used after activation with IL-12, whereas CD8+ DCs were exposed overnight to IFN-γ or CTLA-4–Ig. Groups of CD8+ DCs were also treated with 1-MT or anti–IFN-γ (indicated). Mice deficient in IFN-γ or IFN-γ receptor β chain were also used as a source of CD8+ DCs. *, P < 0.001, experimental versus control footpads. One experiment is reported representative of three. (B) CTLA-4–Ig was examined for its ability to initiate tryptophan catabolism in CD8+ DCs from the same donors as in A. Cells were exposed overnight to CTLA-4–Ig in the presence or absence of anti–IFN-γ, and kynurenine levels were measured in supernatants. *, P < 0.001, presence versus absence of anti–IFN-γ during CTLA-4–Ig exposure. One experiment representative of three is shown. (C) CTLA-4–Ig fails to induce STAT1 phosphorylation in NOD mice deficient in IFN-γ receptor β chain. CD8+ DCs, treated overnight with CTLA-4–Ig, were assayed for STAT1 phosphorylation by Western blot analysis. One of two experiments with similar results is shown.
Figure 4.
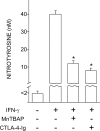
Antagonistic effect of MnTBAP and CTLA-4–Ig on induction of peroxynitrite by IFN-γ in CD8+ DCs from early prediabetic NOD female mice. CD8+ DCs were recovered from these animals to be incubated overnight with IFN-γ. Groups of cells were cotreated with MnTBAP or pretreated with CTLA-4–Ig before exposure to IFN-γ. Nitrotyrosine in cell supernatants was assayed by sandwich ELISA, the lower detection limit of the assay being 2 nM. Data are means ± SD of replicate samples in one of three experiments. *, P < 0.001, combined treatment with MnTBAP or CTLA-4–Ig versus IFN-γ alone.
Figure 5.
Antagonistic effect of 2-MeOE2 on CTLA-4–Ig activity. Early prediabetic NOD female mice were used as a source of CD8+ DCs that were exposed to 2-MeOE2 for 5 h before overnight incubation with CTLA-4–Ig. Nitrotyrosine in cell supernatants was assayed by ELISA or cells were tested in a kynurenine production assay of IDO functional activity, according to conditions specified above. Alternatively, the CD8+ DCs were loaded with NRP-A7, admixed with IL-12–treated and peptide-loaded CD8− DCs, and used in a skin test assay. *, P < 0.001, presence versus absence of 2MeOE2 during CTLA-4–Ig treatment; **, P < 0.001, experimental versus control footpads. One experiment representative of three is shown.
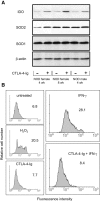
Figure 6.
Ability of CTLA-4–Ig to restore SOD2 expression and function in CD8+ DCs from 4-wk-old NOD female mice. (A) CTLA-4–Ig induces SOD2 and IDO expression. Cells from different types of donor (indicated) were treated overnight with CTLA-4–Ig to be assayed by Western blot. IDO expression was investigated with an IDO-specific polyclonal antibody, whereas SOD2 and SOD1 were analyzed with specific antibody reagents. Loading controls consisted of samples reprobed with β actin–specific antibody. One experiment representative of three is shown. (B) Superoxide accumulation in CD8+ DCs treated with IFN-γ and reversal of the effect by CTLA-4–Ig. Cells were assayed for intracellular superoxide content by flow cytometry after treatment with IFN-γ or sequential exposure to CTLA-4–Ig and IFN-γ (indicated). Cells treated with CTLA-4–Ig alone were also assayed along with cells exposed to 200 μM of a nonspecific inducer of superoxide, H2O2. Control cells (untreated) were freshly harvested DCs. Mean channel fluorescence intensity values are also indicated. One of two experiments is shown.
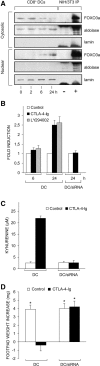
Figure 7.
Ability of CTLA-4–Ig or a PI3K inhibitor to activate FOXO as revealed by different assays. (A) CTLA-4–Ig regulates FOXO expression. Cytoplasmic and nuclear extracts from CD8+ DCs of prediabetic mice were subjected to immunoblot analysis for FOXO3a protein expression. Cells were either untreated (time 0) or treated with CTLA-4–Ig for different times (indicated). The analysis was also conducted on NIH/3T3 cell immunoprecipitates (IP) obtained with control (−) or FOXO3a-specific antibody (+). Controls for fractionation included the use of antibodies specific for the cytosolic marker aldolase and the nuclear marker lamin. These antibodies nonspecifically reacted with the heavy chain of the Igs used for immunoprecipitation of NIH/3T3 cells. One experiment representative of three is shown. (B) CTLA-4–Ig and the PI3K inhibitor activate FOXO-dependent transcription in DCs. CD8+ DCs purified from prediabetic NOD female mice were transfected with pRL-TK in combination with FHRE-Luc. After 24 h, cells were incubated with CTLA-4–Ig or the PI3K inhibitor LY294002. Luciferase activity was monitored at 6 and 24 h. Results are expressed as fold induction (mean ± SD of three independent experiments) of the sample incubated with CTLA-4–Ig or LY294002 versus the corresponding untreated sample, the control value being 1. Also reported in B are values of luciferase activity in siRNA-treated DCs in which the FOXO3a gene had been silenced. In mock-transfected DCs (i.e., cells treated with DOTAP alone), the fold induction at 24 h of CTLA-4–Ig treatment was 2.4 ± 0.3. (C) siRNA-FOXO3a inhibits tryptophan catabolism in DCs treated with CTLA-4–Ig. CD8+ DCs from early prediabetic female mice, either mock-transfected (DC) or treated with siRNA-FOXO3a (DC/siRNA), were exposed overnight to CTLA-4–Ig, and kynurenine levels were measured in supernatants. One experiment representative of two is shown. (D) siRNA-FOXO3a inhibits the tolerogenic potential conferred by CTLA-4–Ig on DCs. Combinations of CD8+ DCs (either mock or siRNA-FOXO3a transfected) and IL-12–treated CD8− DCs were loaded with NRP-A7 and injected into recipient mice to be assayed at 2 wk for skin test reactivity. Either type of CD8+ DC was used after control or CTLA-4–Ig treatment. *, P < 0.001, experimental versus control footpads. One experiment is reported representative of two.
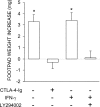
Figure 8.
IFN-γ in combination with a PI3K inhibitor can induce tolerizing properties in CD8+ DCs from early prediabetic NOD female mice. Combinations of CD8+ and IL-12–treated CD8− DCs were loaded with NRP-A7 and injected into recipient mice to be assayed at 2 wk for skin test reactivity. CD8+ DCs were used after exposure to IFN-γ in the presence or absence of LY294002. In an alternative, CD8+ DCs were treated with CTLA-4–Ig. *, P < 0.001, experimental versus control footpads. One experiment is reported representative of three.
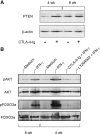
Figure 9.
Effect of CTLA-4–Ig alone or in combination with IFN-γ on the expression of PTEN, AKT/phospho-AKT, and FOXO3a/phospho-FOXO3a in early prediabetic NOD female mice. (A) Defective PTEN expression and reversal by CTLA-4–Ig in 4-wk-old mice. CD8+ DCs from female donors of different ages were treated overnight with CTLA-4–Ig before Western blot analysis. PTEN expression was investigated with a specific monoclonal antibody. Loading controls consisted of samples reprobed with β actin–specific antibody. One experiment representative of three is shown. (B) Enhanced phosphorylation of AKT and FOXO3a in response to a 30-min activation with IFN-γ in the same cells as in A and reversal by pretreatment with CTLA-4–Ig (18 h) or LY294002 (5 h). CD8+ DCs from 8-wk-old NOD females were used as control.
Figure 10.
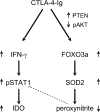
Hypothetical model for the role of CTLA-4–Ig in correcting the defective tolerogenesis of early prediabetic NOD female mice. CTLA-4–Ig may induce IFN-γ production in CD8+ DCs and, at the same time, enable cell responsiveness to the autocrine STAT1-dependent effects of the cytokine, leading to activation of the tolerogenic pathway of tryptophan catabolism. Although aberrant peroxynitrite formation would normally impede STAT1 phosphorylation and IFN-γ responsiveness in diabetes-prone animals (dotted line), CTLA-4–Ig may reduce peroxynitrite formation via enhanced SOD2 expression. This, in turn, could result from increased activity of the transcription factor FOXO3a, whose nuclear persistence is conditioned by positive and negative regulators such as PTEN and phospho-AKT, both of which appear to be affected by cell treatment with CTLA-4–Ig.
Similar articles
- A defect in tryptophan catabolism impairs tolerance in nonobese diabetic mice.
Grohmann U, Fallarino F, Bianchi R, Orabona C, Vacca C, Fioretti MC, Puccetti P. Grohmann U, et al. J Exp Med. 2003 Jul 7;198(1):153-60. doi: 10.1084/jem.20030633. Epub 2003 Jun 30. J Exp Med. 2003. PMID: 12835483 Free PMC article. - CTLA-4-Ig regulates tryptophan catabolism in vivo.
Grohmann U, Orabona C, Fallarino F, Vacca C, Calcinaro F, Falorni A, Candeloro P, Belladonna ML, Bianchi R, Fioretti MC, Puccetti P. Grohmann U, et al. Nat Immunol. 2002 Nov;3(11):1097-101. doi: 10.1038/ni846. Epub 2002 Sep 30. Nat Immunol. 2002. PMID: 12368911 - Cutting edge: silencing suppressor of cytokine signaling 3 expression in dendritic cells turns CD28-Ig from immune adjuvant to suppressant.
Orabona C, Belladonna ML, Vacca C, Bianchi R, Fallarino F, Volpi C, Gizzi S, Fioretti MC, Grohmann U, Puccetti P. Orabona C, et al. J Immunol. 2005 Jun 1;174(11):6582-6. doi: 10.4049/jimmunol.174.11.6582. J Immunol. 2005. PMID: 15905495 - CD40 ligation prevents onset of tolerogenic properties in human dendritic cells treated with CTLA-4-Ig.
Vacca C, Fallarino F, Perruccio K, Orabona C, Bianchi R, Gizzi S, Velardi A, Fioretti MC, Puccetti P, Grohmann U. Vacca C, et al. Microbes Infect. 2005 Jun;7(7-8):1040-8. doi: 10.1016/j.micinf.2005.03.030. Microbes Infect. 2005. PMID: 15925532 - Restoring functions of tumor suppressors with small molecules.
Smukste I, Stockwell BR. Smukste I, et al. Cancer Cell. 2003 Dec;4(6):419-20. doi: 10.1016/s1535-6108(03)00307-6. Cancer Cell. 2003. PMID: 14706331 Review.
Cited by
- Immune suppression in the tumor microenvironment: a role for dendritic cell-mediated tolerization of T cells.
Hurwitz AA, Watkins SK. Hurwitz AA, et al. Cancer Immunol Immunother. 2012 Feb;61(2):289-293. doi: 10.1007/s00262-011-1181-5. Epub 2012 Jan 12. Cancer Immunol Immunother. 2012. PMID: 22237887 Free PMC article. Review. - Molecular control of steady-state dendritic cell maturation and immune homeostasis.
Hammer GE, Ma A. Hammer GE, et al. Annu Rev Immunol. 2013;31:743-91. doi: 10.1146/annurev-immunol-020711-074929. Epub 2013 Jan 17. Annu Rev Immunol. 2013. PMID: 23330953 Free PMC article. Review. - FoxO Transcription Factors and Regenerative Pathways in Diabetes Mellitus.
Maiese K. Maiese K. Curr Neurovasc Res. 2015;12(4):404-13. doi: 10.2174/1567202612666150807112524. Curr Neurovasc Res. 2015. PMID: 26256004 Free PMC article. Review. - Diabetes mellitus: channeling care through cellular discovery.
Maiese K, Shang YC, Chong ZZ, Hou J. Maiese K, et al. Curr Neurovasc Res. 2010 Feb;7(1):59-64. doi: 10.2174/156720210790820217. Curr Neurovasc Res. 2010. PMID: 20158461 Free PMC article. - Intrinsic and extrinsic control of peripheral T-cell tolerance by costimulatory molecules of the CD28/ B7 family.
Bour-Jordan H, Esensten JH, Martinez-Llordella M, Penaranda C, Stumpf M, Bluestone JA. Bour-Jordan H, et al. Immunol Rev. 2011 May;241(1):180-205. doi: 10.1111/j.1600-065X.2011.01011.x. Immunol Rev. 2011. PMID: 21488898 Free PMC article. Review.
References
- Atkinson, M.A., and E.H. Leiter. 1999. The NOD mouse model of type 1 diabetes: as good as it gets? Nat. Med. 5:601–604. - PubMed
- Serreze, D.V., H.R. Gaskins, and E.H. Leiter. 1993. Defects in the differentiation and function of antigen presenting cells in NOD/Lt mice. J. Immunol. 150:2534–2543. - PubMed
- Suarez-Pinzon, W.L., J.G. Mabley, K. Strynadka, R.F. Power, C. Szabo, and A. Rabinovitch. 2001. An inhibitor of inducible nitric oxide synthase and scavenger of peroxynitrite prevents diabetes development in NOD mice. J. Autoimmun. 16:449–455. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous