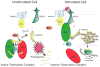Glycogen synthase kinase-3 in insulin and Wnt signalling: a double-edged sword? - PubMed (original) (raw)
Review
Glycogen synthase kinase-3 in insulin and Wnt signalling: a double-edged sword?
S Patel et al. Biochem Soc Trans. 2004 Nov.
Abstract
Glycogen synthase kinase-3 is an unusual protein serine/threonine kinase that, unlike most of its 500-odd relatives in the genome, is active under resting conditions and is inactivated upon cell stimulation. The two mammalian isoforms, GSK-3alpha and beta, play largely overlapping roles and have been implicated in a variety of human pathologies, including Type II diabetes, Alzheimer's disease, bipolar disorder and cancer. Recently, the modes of regulation of this enzyme have been elucidated through a combination of structural and cell biological studies. A series of relatively selective small molecules have facilitated chemical manipulation of the enzyme in intact cells and tissues, and new roles for the protein kinase in embryonic stem cell differentiation and motility have emerged. Despite these advances, the therapeutic value of this enzyme as a drug target remains clouded by uncertainty over the potential of antagonists to promote tumorigenesis. This article describes the state of understanding of this intriguing enzyme, and weighs current evidence regarding whether there is a therapeutic window for amelioration of diseases in which it is implicated, in the absence of inducing new pathologies.
Figures
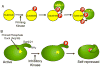
Fig. 1
A. GSK-3 preferentially phosphorylates substrates that are pre-phosphorylated by a priming kinase. For example, in the case of the GSK-3 substrate β-catenin, phosphorylation by CKI on S45 primes it for sequential phosphorylation on T41, S37 and S33 by GSK-3. B. Pseudosubstrate inactivation of GSK-3. The N-termini of GSK-3α and β contain serine residues (S21 and S9, respectively) that when phosphorylated by an inhibitory kinase, serve as primed-pseudosubstrates that can occupy the substrate binding pocket of GSK-3, inhibiting its activity towards true substrates.
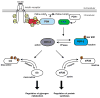
Figure 2. Regulation of GSK-3 by the insulin-signal transduction pathway
The binding of insulin to its cell surface receptor triggers the recruitment and activation of PI3K. At the plasma membrane, PI3K stimulates the formation of phosphatidylinositol-3,4,5trisphosphate (PtdIns (3,4,5)P3) which triggers the colocalisation of phosphoinositide dependent kinase 1 (PDK1) and PKB allowing PDK1 to activate PKB. Upon activation, PKB phosphorylates and inactivates GSK-3 resulting in the dephosphorylation of glycogen synthase and eIF2B, two substrates of GSK-3 that control the rates of glycogen metabolism and protein synthesis respectively.
Fig. 3. Central Role of GSK-3 in the Wnt/β-catenin Pathway
In unstimulated cells, a minimal complex of GSK-3, axin, APC and β-catenin is required for GSK-3-mediated phosphorylation of β-catenin which targets it for ubiqitination and degradation. Wnt stimulation activates the receptor Frizzled, and co-receptor LRP5/6, that then signal through Dishevelled, using an unclear mechanism, to inactivate β-catenin phosphorylation. Unphosphorylated β-catenin accumulates and translocates to the nucleus where it transactivates genes regulated by TCF/LEF transcription factors.
Similar articles
- [A new target for diabetes therapy: advances in the research of glycogen synthase kinase-3 inhibitors].
Liu SN, Shen ZF. Liu SN, et al. Yao Xue Xue Bao. 2007 Dec;42(12):1227-31. Yao Xue Xue Bao. 2007. PMID: 18338632 Review. Chinese. - Glycogen synthase kinase 3: a key regulator of cellular fate.
Forde JE, Dale TC. Forde JE, et al. Cell Mol Life Sci. 2007 Aug;64(15):1930-44. doi: 10.1007/s00018-007-7045-7. Cell Mol Life Sci. 2007. PMID: 17530463 Free PMC article. Review. - Expression profiling and functional analysis of wnt signaling mechanisms in mesenchymal stem cells.
Etheridge SL, Spencer GJ, Heath DJ, Genever PG. Etheridge SL, et al. Stem Cells. 2004;22(5):849-60. doi: 10.1634/stemcells.22-5-849. Stem Cells. 2004. PMID: 15342948 - Glycogen Synthase Kinase 3: A Kinase for All Pathways?
Patel P, Woodgett JR. Patel P, et al. Curr Top Dev Biol. 2017;123:277-302. doi: 10.1016/bs.ctdb.2016.11.011. Epub 2016 Dec 28. Curr Top Dev Biol. 2017. PMID: 28236969 Review.
Cited by
- Alcohol-induced ketonemia is associated with lowering of blood glucose, downregulation of gluconeogenic genes, and depletion of hepatic glycogen in type 2 diabetic db/db mice.
Srinivasan MP, Shawky NM, Kaphalia BS, Thangaraju M, Segar L. Srinivasan MP, et al. Biochem Pharmacol. 2019 Feb;160:46-61. doi: 10.1016/j.bcp.2018.12.005. Epub 2018 Dec 7. Biochem Pharmacol. 2019. PMID: 30529690 Free PMC article. - The paradoxical pro- and anti-apoptotic actions of GSK3 in the intrinsic and extrinsic apoptosis signaling pathways.
Beurel E, Jope RS. Beurel E, et al. Prog Neurobiol. 2006 Jul;79(4):173-89. doi: 10.1016/j.pneurobio.2006.07.006. Epub 2006 Aug 28. Prog Neurobiol. 2006. PMID: 16935409 Free PMC article. Review. - GSK-3beta controls osteogenesis through regulating Runx2 activity.
Kugimiya F, Kawaguchi H, Ohba S, Kawamura N, Hirata M, Chikuda H, Azuma Y, Woodgett JR, Nakamura K, Chung UI. Kugimiya F, et al. PLoS One. 2007 Sep 5;2(9):e837. doi: 10.1371/journal.pone.0000837. PLoS One. 2007. PMID: 17786208 Free PMC article. - Equine Metabolic Syndrome: A Complex Disease Influenced by Multifactorial Genetic Factors.
Stefaniuk-Szmukier M, Piórkowska K, Ropka-Molik K. Stefaniuk-Szmukier M, et al. Genes (Basel). 2023 Jul 27;14(8):1544. doi: 10.3390/genes14081544. Genes (Basel). 2023. PMID: 37628596 Free PMC article. Review. - GSK-3beta in mouse fibroblasts controls wound healing and fibrosis through an endothelin-1-dependent mechanism.
Kapoor M, Liu S, Shi-wen X, Huh K, McCann M, Denton CP, Woodgett JR, Abraham DJ, Leask A. Kapoor M, et al. J Clin Invest. 2008 Oct;118(10):3279-90. doi: 10.1172/JCI35381. J Clin Invest. 2008. PMID: 18802478 Free PMC article. Retracted.
References
- Embi N, Rylatt DB, Cohen P. Eur J Biochem. 1980;107:519–27. - PubMed
- Parker PJ, Caudwell FB, Cohen P. Eur J Biochem. 1983;130:227–34. - PubMed
- Ramakrishna S, Benjamin WB. J Biol Chem. 1988;263:12677–81. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical