Rho GTPases and leucocyte-induced endothelial remodelling - PubMed (original) (raw)
Review
Rho GTPases and leucocyte-induced endothelial remodelling
Jaime Millán et al. Biochem J. 2005.
Abstract
Leucocytes in the bloodstream respond rapidly to inflammatory signals by crossing the blood vessel wall and entering the tissues. This process involves adhesion to, and subsequent transmigration across, the endothelium, mediated by a cascade of interactions between adhesion molecules and stimulation of intracellular signalling pathways in both leucocytes and endothelial cells. This leads to changes in endothelial cell morphology that assist leucocyte extravasation, including endothelial cell contraction, intercellular junction disruption, increased permeability, remodelling of the endothelial apical surface and alterations in vesicle trafficking. Rho GTPases play a central role in many of the endothelial responses to leucocyte interaction. In this review, we discuss recent findings on leucocyte-induced alterations to endothelial cells, and the roles of Rho GTPases in these responses.
Figures
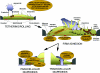
Figure 1. Possible remodelling of the endothelium caused by leucocyte interaction
Based on signalling induced by ligation (either by leucocytes or by specific antibodies) of endothelial receptors involved in each step of the cascade of transmigration, leucocytes may alter the endothelium in several ways. During the initial tethering and rolling, E-selectin may transiently signal to the actin cytoskeleton and redistribute caveolae. During firm adhesion, the turnover of focal adhesions may be altered and ICAM-1- and VCAM-1-mediated signalling may increase actomyosin contractility. VCAM-1 would also cause disruption of adherens junctions. During TEM, PECAM-1 could be translocated from an internal compartment to the plasma membrane. Simultaneously, a haptotactic gradient of chemokines may guide the leucocyte to the VVOs formed by caveolae. Each of these processes may guide the leucocyte towards either a paracellular or a transcellular route for TEM.
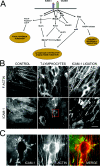
Figure 2. The roles of RhoA and Rac in leucocyte-induced signalling to the endothelium
(A) ICAM-1, and probably VCAM-1, ligation induces RhoA activation and triggers the formation of stress fibres and actomyosin contractility by increasing the phosphorylation of MLC. In addition, VCAM-1 clustering induces Rac1-mediated generation of ROS via NADPH oxidase, which disrupts adherens junctions. Phosphorylation of FAK, paxillin and Cas proteins induced by ICAM-1 cross-linking may contribute to alter the turnover of endothelial focal adhesions. (B) T-lymphoblasts or ICAM-1 ligation induce RhoA-mediated stress fibre formation. HUVECs were incubated with T-lymphoblasts or clustered with anti-ICAM-1 specific antibodies for 45 min, and ICAM-1 and F-actin were detected with an anti-ICAM-1 antibody or phalloidin respectively. Bar, 20 μm. (C) A 4-fold magnification of the squared area in (B) is shown. Transmigratory T-lymphoblast is surrounded by a microvillus-like docking structure. Despite being enriched in adhesion receptors involved in leucocyte firm adhesion, abrogation of these structures inhibits leucocyte TEM, but not adhesion [12]. ROCK may mediate the formation of these docking complexes [11]. Images in (B) and (C) were taken with a coolview 12-bit integrating cooled CCD camera (Photonic Science, Robertsbridge, East Sussex, U.K.) mounted over an Axiophot microscope (Carl Zeiss Ltd, Welwyn Garden City, Herts., U.K.).
Similar articles
- Rho GTPases and leukocyte adhesion receptor expression and function in endothelial cells.
Cernuda-Morollón E, Ridley AJ. Cernuda-Morollón E, et al. Circ Res. 2006 Mar 31;98(6):757-67. doi: 10.1161/01.RES.0000210579.35304.d3. Circ Res. 2006. PMID: 16574916 Review. - An in vitro model to study the role of endothelial rho GTPases during leukocyte transendothelial migration.
Millán J, Williams L, Ridley AJ. Millán J, et al. Methods Enzymol. 2006;406:643-55. doi: 10.1016/S0076-6879(06)06050-2. Methods Enzymol. 2006. PMID: 16472694 - Rho GTPases and regulation of hematopoietic stem cell localization.
Williams DA, Zheng Y, Cancelas JA. Williams DA, et al. Methods Enzymol. 2008;439:365-93. doi: 10.1016/S0076-6879(07)00427-2. Methods Enzymol. 2008. PMID: 18374178 Review. - Vascular and epithelial junctions: a barrier for leucocyte migration.
Garrido-Urbani S, Bradfield PF, Lee BP, Imhof BA. Garrido-Urbani S, et al. Biochem Soc Trans. 2008 Apr;36(Pt 2):203-11. doi: 10.1042/BST0360203. Biochem Soc Trans. 2008. PMID: 18363562 Review. - The regulation of leucocyte transendothelial migration by endothelial signalling events.
Fernandez-Borja M, van Buul JD, Hordijk PL. Fernandez-Borja M, et al. Cardiovasc Res. 2010 May 1;86(2):202-10. doi: 10.1093/cvr/cvq003. Epub 2010 Jan 12. Cardiovasc Res. 2010. PMID: 20068003 Review.
Cited by
- p120-Catenin prevents neutrophil transmigration independently of RhoA inhibition by impairing Src dependent VE-cadherin phosphorylation.
Alcaide P, Martinelli R, Newton G, Williams MR, Adam A, Vincent PA, Luscinskas FW. Alcaide P, et al. Am J Physiol Cell Physiol. 2012 Aug 15;303(4):C385-95. doi: 10.1152/ajpcell.00126.2012. Epub 2012 May 30. Am J Physiol Cell Physiol. 2012. PMID: 22648953 Free PMC article. - ICAM-1 nanoclusters regulate hepatic epithelial cell polarity by leukocyte adhesion-independent control of apical actomyosin.
Cacho-Navas C, López-Pujante C, Reglero-Real N, Colás-Algora N, Cuervo A, Conesa JJ, Barroso S, de Rivas G, Ciordia S, Paradela A, D'Agostino G, Manzo C, Feito J, Andrés G, Molina-Jiménez F, Majano P, Correas I, Carazo JM, Nourshargh S, Huch M, Millán J. Cacho-Navas C, et al. Elife. 2024 Apr 10;12:RP89261. doi: 10.7554/eLife.89261. Elife. 2024. PMID: 38597186 Free PMC article. - H-Ras, R-Ras, and TC21 differentially regulate ureteric bud cell branching morphogenesis.
Pozzi A, Coffa S, Bulus N, Zhu W, Chen D, Chen X, Mernaugh G, Su Y, Cai S, Singh A, Brissova M, Zent R. Pozzi A, et al. Mol Biol Cell. 2006 Apr;17(4):2046-56. doi: 10.1091/mbc.e05-08-0800. Epub 2006 Feb 8. Mol Biol Cell. 2006. PMID: 16467383 Free PMC article. - Lymphocyte recruitment to the liver: molecular insights into the pathogenesis of liver injury and hepatitis.
Shetty S, Lalor PF, Adams DH. Shetty S, et al. Toxicology. 2008 Dec 30;254(3):136-46. doi: 10.1016/j.tox.2008.08.003. Epub 2008 Aug 19. Toxicology. 2008. PMID: 18775762 Free PMC article. Review. - MYADM controls endothelial barrier function through ERM-dependent regulation of ICAM-1 expression.
Aranda JF, Reglero-Real N, Marcos-Ramiro B, Ruiz-Sáenz A, Fernández-Martín L, Bernabé-Rubio M, Kremer L, Ridley AJ, Correas I, Alonso MA, Millán J. Aranda JF, et al. Mol Biol Cell. 2013 Feb;24(4):483-94. doi: 10.1091/mbc.E11-11-0914. Epub 2012 Dec 21. Mol Biol Cell. 2013. PMID: 23264465 Free PMC article.
References
- Butcher E. C. Leukocyte–endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. - PubMed
- Muller W. A. Leukocyte–endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 2003;24:327–334. - PubMed
- Ostermann G., Weber K. S., Zernecke A., Schroder A., Weber C. JAM-1 is a ligand of the beta(2) integrin LFA-1 involved in transendothelial migration of leukocytes. Nat. Immunol. 2002;3:151–158. - PubMed
- Schenkel A. R., Mamdouh Z., Chen X., Liebman R. M., Muller W. A. CD99 plays a major role in the migration of monocytes through endothelial junctions. Nat. Immunol. 2002;3:143–150. - PubMed
- Vicente-Manzanares M., Sanchez-Madrid F. Role of the cytoskeleton during leukocyte responses. Nat. Rev. Immunol. 2004;4:110–122. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources