Neural crest stem cells undergo multilineage differentiation in developing peripheral nerves to generate endoneurial fibroblasts in addition to Schwann cells - PubMed (original) (raw)
. 2004 Nov;131(22):5599-612.
doi: 10.1242/dev.01429. Epub 2004 Oct 20.
Affiliations
- PMID: 15496445
- PMCID: PMC2638001
- DOI: 10.1242/dev.01429
Neural crest stem cells undergo multilineage differentiation in developing peripheral nerves to generate endoneurial fibroblasts in addition to Schwann cells
Nancy M Joseph et al. Development. 2004 Nov.
Abstract
Neural crest stem cells (NCSCs) persist in peripheral nerves throughout late gestation but their function is unknown. Current models of nerve development only consider the generation of Schwann cells from neural crest, but the presence of NCSCs raises the possibility of multilineage differentiation. We performed Cre-recombinase fate mapping to determine which nerve cells are neural crest derived. Endoneurial fibroblasts, in addition to myelinating and non-myelinating Schwann cells, were neural crest derived, whereas perineurial cells, pericytes and endothelial cells were not. This identified endoneurial fibroblasts as a novel neural crest derivative, and demonstrated that trunk neural crest does give rise to fibroblasts in vivo, consistent with previous studies of trunk NCSCs in culture. The multilineage differentiation of NCSCs into glial and non-glial derivatives in the developing nerve appears to be regulated by neuregulin, notch ligands, and bone morphogenic proteins, as these factors are expressed in the developing nerve, and cause nerve NCSCs to generate Schwann cells and fibroblasts, but not neurons, in culture. Nerve development is thus more complex than was previously thought, involving NCSC self-renewal, lineage commitment and multilineage differentiation.
Figures
Fig. 1
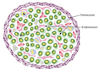
Peripheral nerve anatomy. Perineurial cells form a sheath around the nerve (the perineurium; purple) that separates the endoneurial environment from the epineurial environment (outside the nerve bundle). Within the endoneurium, there are Schwann cells that myelinate axons (S; green cells surrounding yellow axons), non-myelinating Schwann cells that are associated with multiple axons (N; blue cells), endoneurial fibroblasts (F; pink cells), and pericytes (P; red) that surround small blood vessels. A single layer of endothelial cells also surrounds the blood vessels (not shown). Perineurial cells are distinguished based on their position and their characteristic flattened morphology. Pericytes are distinguished by their expression of SMA. Schwann cells are distinguished by their association with axons, their expression of glial markers such as S100β, and their basal lamina. Endoneurial fibroblasts lack a basal lamina, often have long interdigitating processes, are not associated with axons, and fail to express SMA or S100β in vivo.
Fig. 2
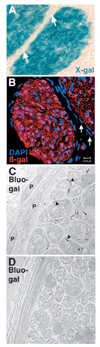
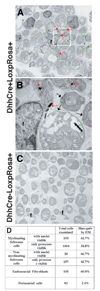
Perineurial cells are not neural crest derived. Sciatic nerves from Wnt1-Cre+loxpRosa+ mouse pups were dissected and processed for X-gal staining (A), immunohistochemistry (B), or electron microscopy (C,D), at either postnatal day 3 (A) or 11 (B–D). According to each technique the perineurial cells failed to express β-gal and therefore were not neural crest derived. Sections through X-gal stained (A) or anti-β-gal antibody stained (B) nerves consistently exhibited strong staining throughout the endoneurial space, but the flattened layers of perineurial cells that distinguish different bundles of axons were notable for their lack of X-gal or anti-β-gal antibody staining (arrows, A,B). We also failed to observe X-gal staining in perineurial cells from P0-Cre+β-actin-lacZ+ mice or Wnt1-Cre+β-actin-lacZ+ mice (data not shown). Note that the red fluorescent cell in the perineurial layer (bottom right corner; B) was an autofluorescent blood cell within a blood vessel (see Fig. S4 in supplementary material). Bluo-gal forms an electron dense precipitate that is visible as small black crystals (arrowheads, C) by electron microscopy after digestion by β-galactosidase (Weis et al., 1991). Bluo-gal staining was consistently absent from perineurial cells (P; panel C), but was visible in most Schwann cells (to the right of the perineurial cells in panel C). The myelin sheaths within the Schwann cells appear light and fragmented because bluo-gal staining required fixation conditions that did not effectively preserve myelin. Note the lack of bluo-gal staining in a nerve section from a control littermate (D).
Fig. 3
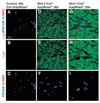
Pericytes and endothelial cells within the sciatic nerve are not neural crest derived. Sciatic nerves were dissected from P11 Wnt1-Cre−loxpRosa+ and Wnt1-Cre+loxpRosa+ pups, and immunohistochemically stained for β-gal (green), the endothelial marker PECAM1 (blue), and the pericyte marker SMA (red). Although most cells in the endoneurial space were β-gal+, both the PECAM1+ endothelial cells and the SMA+ pericytes consistently failed to co-label with β-gal.
Fig. 4
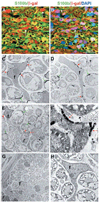
Endoneurial fibroblasts are neural crest derived. Sciatic nerves were dissected from P11 Wnt1-Cre+loxpRosa+ pups and processed for immunohistochemistry (A,B), or electron microscopy (C–H). Nerves were stained with antibodies against β-gal (red) to identify neural crest-derived cells, and S100β (green) to identify Schwann cells. The same field of view is shown in A and B, with DAPI staining (blue) added to panel B. In addition to β-gal+S100β+ glia (green or yellow), we also observed β-gal+S100β− endoneurial cells (white arrows) that were neither associated with axons nor blood vessels. By electron microscopy, we consistently observed endoneurial fibroblasts stained with bluo-gal, indicating neural crest-derivation (C–F). (C,D) Bluo-gal-stained endoneurial fibroblasts (f), surrounded by myelinating Schwann cells, most of which are also stained with bluo-gal. Red arrows indicate bluo-gal crystals in the fibroblast; green arrows indicate bluo-gal crystals in Schwann cells. (E) A lower magnification image of a large bluo-gal stained fibroblast, with a very long process, surrounded by Schwann cells. The boxed area is shown at a higher magnification in F. Note the presence of a basal lamina around the Schwann cells (black arrows), but the lack of a basal lamina around the fibroblast process. (G,H) Low and high power images of sections through control littermates that lack any bluo-gal staining in perineurial cells (G), Schwann cells (G,H) or fibroblasts (H).
Fig. 5
Dhh-Cre expressing progenitors give rise to endoneurial fibroblasts as well as Schwann cells in nerves. Sciatic nerves were dissected from P11 Dhh-Cre+LoxpRosa+ (A,B) pups as well as Dhh-Cre−LoxpRosa+ (C) littermate controls, processed for bluo-gal staining, and examined by electron microscopy. Endoneurial fibroblasts (f) were identified by their angular shape, long processes (A), and lack of a basal lamina (B). Red arrows indicate bluo-gal crystals in an endoneurial fibroblast; green arrows indicate bluo-gal crystals in Schwann cells. B shows the inset from panel A at a higher magnification to demonstrate the presence of a basal lamina on Schwann cells (black arrows), and the absence of a basal lamina on the fibroblast. Bluo-gal crystals were never observed in littermate control nerves (C). Endoneurial fibroblasts expressed β-gal at a similar frequency to Schwann cells, whereas perineurial cells were nearly all negative (D).
Fig. 6
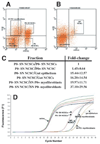
Dhh expression by progenitor populations in the developing nerve. We isolated p75+P0 − NCSCs (A; 83% of these cells formed multilineage colonies, and 6% formed colonies that contained myofibroblasts and glia in a clonal analysis), p75+P0 + NCSCs (A; 74% of these cells formed multilineage colonies, and 12% formed myofibroblasts and glia), p75−P0 + myofibroblast progenitors (A; 97% of these cells formed myofibroblast-only colonies) and p75−P0 − myofibroblast progenitors (A; 99% of these cells formed myofibroblast-only colonies) from E14.5 rat sciatic nerve (Morrison et al., 1999). Only small numbers of restricted glial progenitors (that form glial-only colonies in culture) are present in the E14.5 sciatic nerve, but the frequency of these cells increases from E15.5 to E17.5, as NCSCs undergo glial restriction (Morrison et al., 1999). As negative controls, we also isolated p75+α4+ gut NCSCs (B; 76% of these cells formed multilineage colonies) and p75−α4− gut epithelial progenitors (B; none of these cells gave rise to colonies that contained neural cells, although they did give rise to fibroblasts in culture). By quantitative (real-time) RT-PCR, we found that nerve p75+P0 − NCSCs expressed the highest levels of Dhh, followed by slightly lower levels in p75+P0 + NCSCs (1.45-fold lower), gut epithelial cells (15-fold lower), gut NCSCs (16-fold lower), and nerve myofibroblasts (20 to 40-fold lower) (C). As no reporter gene expression was observed within the intestines of Dhh-Cre+loxpRosa+ mice, the low level of Dhh expression in nerve myofibroblasts appears to be inconsistent with reporter gene activation within these cells. D shows a representative experiment in which cDNA was random primed and normalized based on β-actin content, and then Dhh expression levels were compared between samples. We confirmed that each reaction product was homogeneous based on the melting curve, and that a single product was generated of the expected size (data not shown). Sequencing of the Dhh band confirmed that the correct sequence had been amplified.
Fig. 7
Some myofibroblast progenitors are neural crest derived. Wnt1-Cre mice were mated with β-actin-flox/stop-β-gal conditional reporter mice. Sciatic nerves were dissected from the fetuses at E13.5, dissociated and cultured at clonal density. Some cells formed mixed colonies containing neural cells as well as myofibroblasts (A), whereas other colonies contained only neural cells (B), or only myofibroblasts (C). Neural cells were p75+ (not shown), had a characteristically spindly morphology, and grew in relatively dense colonies (B). Myofibroblasts were p75−SMA+, were characteristically large and flat, and grew in small, dispersed colonies (C,D). The cultures were stained with X-gal to identify neural crest-derived cells (blue product). Colonies from Cre−reporter+ littermate controls were never X-gal+ (A; Table 2), but neural colonies from Cre+reporter+ mice were usually X-gal+ (B; Table 2), as would be expected. Myofibroblast colonies from Cre+reporter+ mice were sometimes X-gal+, indicating neural crest derivation (C). Similar results were obtained using Wnt1-Cre+loxpRosa+ mice, in which some nerve cells formed β-gal+SMA+ myofibroblast colonies (D; see Table 2 for quantification).
Fig. 8
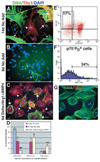
NCSC-derived myofibroblasts express the endoneurial fibroblast marker Thy1, and the generation of these cells is enhanced by Bmp4, Nrg1 and Delta. (A,D) After 14 days in standard medium, nerve NCSCs give rise to myofibroblasts that are either SMA+Thy1− (purple arrows; 46±17% of cells with myofibroblast morphology), SMA+Thy1+ (white arrows; 44±15%), or SMA−Thy1+ (white arrowhead; 10±3%). Note that these colonies also contain large numbers of neurons and glia that are not visible in this field of view. After only 6 days in standard medium (B,D), NCSCs give rise to colonies containing mainly undifferentiated cells, as well as small numbers of myofibroblasts, which are mostly (92±6%) SMA+Thy1− (purple arrows). (C,D) In 6 day cultures supplemented with Delta, Nrg1 and Bmp4, nerve NCSCs give rise to larger numbers of myofibroblast cells, that include a higher frequency of SMA+Thy1+ cells (white arrows; 60±8%), and SMA−Thy1+ cells (white arrowhead; 28±7%) and a lower frequency of SMA+Thy1− cells (not shown; 12±4%). *P<0.05, relative to 6 day No Add (standard medium without Delta, Nrg1 or Bmp4 added);∂ indicates P<0.05, relative to 14 day No add (D). (E) Neural crest-derived myofibroblast progenitors can be isolated from acutely dissociated fetal rat sciatic nerve as p75−P0 + cells. (F) On average, 40±11% of these p75−P0 + cells express Thy1 in vivo. (G) 100% of the colonies that arose in culture from these p75−P0+Thy1+ cells were myofibroblast-only, and about 83% of these colonies consisted exclusively of SMA+Thy1− cells.
Fig. 9
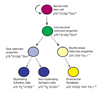
Model of peripheral nerve development. NCSCs self-renew in peripheral nerves to generate additional NCSCs (Morrison et al., 1999), as well as undergoing restriction under the influence of Nrg1, Delta and Bmp4. The resulting restricted progenitors lack neuronal potential (non-neuronal) but undergo lineage commitment to form both glial and myofibroblast progenitors. Glial progenitors differentiate to form both myelinating and non-myelinating Schwann cells, and myofibroblast progenitors differentiate to form endoneurial fibroblasts. It is likely that Nrg1, Delta and Bmp4 play multiple roles by having different effects on different cells within these lineages. For example, Nrg1 promotes the survival and glial restriction of NCSCs (Morrison et al., 1999; Shah et al., 1994), whereas it promotes the survival, proliferation and maturation of glial progenitors/Schwann cells (Dong et al., 1995), and the proliferation but not the survival of myofibroblast progenitors (Morrison et al., 1999).
Similar articles
- Cell-intrinsic differences between stem cells from different regions of the peripheral nervous system regulate the generation of neural diversity.
Bixby S, Kruger GM, Mosher JT, Joseph NM, Morrison SJ. Bixby S, et al. Neuron. 2002 Aug 15;35(4):643-56. doi: 10.1016/s0896-6273(02)00825-5. Neuron. 2002. PMID: 12194865 - Neural crest stem cells persist in the adult gut but undergo changes in self-renewal, neuronal subtype potential, and factor responsiveness.
Kruger GM, Mosher JT, Bixby S, Joseph N, Iwashita T, Morrison SJ. Kruger GM, et al. Neuron. 2002 Aug 15;35(4):657-69. doi: 10.1016/s0896-6273(02)00827-9. Neuron. 2002. PMID: 12194866 Free PMC article. - The trunk neural crest and its early glial derivatives: a study of survival responses, developmental schedules and autocrine mechanisms.
Woodhoo A, Dean CH, Droggiti A, Mirsky R, Jessen KR. Woodhoo A, et al. Mol Cell Neurosci. 2004 Jan;25(1):30-41. doi: 10.1016/j.mcn.2003.09.006. Mol Cell Neurosci. 2004. PMID: 14962738 - [Phenotypic plasticity of neural crest-derived melanocytes and Schwann cells].
Dupin E. Dupin E. Biol Aujourdhui. 2011;205(1):53-61. doi: 10.1051/jbio/2011008. Epub 2011 Apr 19. Biol Aujourdhui. 2011. PMID: 21501576 Review. French. - Development of the Schwann cell lineage: from the neural crest to the myelinated nerve.
Woodhoo A, Sommer L. Woodhoo A, et al. Glia. 2008 Nov 1;56(14):1481-1490. doi: 10.1002/glia.20723. Glia. 2008. PMID: 18803317 Review.
Cited by
- Novel Glial Cell Functions: Extensive Potency, Stem Cell-Like Properties, and Participation in Regeneration and Transdifferentiation.
Milichko V, Dyachuk V. Milichko V, et al. Front Cell Dev Biol. 2020 Aug 18;8:809. doi: 10.3389/fcell.2020.00809. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33015034 Free PMC article. Review. - On the road again: Establishment and maintenance of stemness in the neural crest from embryo to adulthood.
Perera SN, Kerosuo L. Perera SN, et al. Stem Cells. 2021 Jan;39(1):7-25. doi: 10.1002/stem.3283. Epub 2020 Nov 4. Stem Cells. 2021. PMID: 33017496 Free PMC article. Review. - BMP4 and Neuregulin regulate the direction of mouse neural crest cell differentiation.
Zhu S, Liu W, Ding HF, Cui H, Yang L. Zhu S, et al. Exp Ther Med. 2019 May;17(5):3883-3890. doi: 10.3892/etm.2019.7439. Epub 2019 Mar 26. Exp Ther Med. 2019. PMID: 31007733 Free PMC article. - Constitutively active Notch1 converts cranial neural crest-derived frontonasal mesenchyme to perivascular cells in vivo.
Miller SR, Perera SN, Baker CV. Miller SR, et al. Biol Open. 2017 Mar 15;6(3):317-325. doi: 10.1242/bio.023887. Biol Open. 2017. PMID: 28183698 Free PMC article. - Mouse embryonic dorsal root ganglia contain pluripotent stem cells that show features similar to embryonic stem cells and induced pluripotent stem cells.
Ogawa R, Fujita K, Ito K. Ogawa R, et al. Biol Open. 2017 May 15;6(5):602-618. doi: 10.1242/bio.021758. Biol Open. 2017. PMID: 28373172 Free PMC article.
References
- Anderson DJ. Stem cells and pattern formation in the nervous system: the possible versus the actual. Neuron. 2001;30:19–35. - PubMed
- Assouline JG, Bosch EP, Lim R. Purification of rat Schwann cells from cultures of peripheral nerve: an immunoselective method using surfaces coated with anti-immunoglobulin antibodies. Brain Res. 1983;277:389–392. - PubMed
- Bitgood MJ, McMahon AP. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev. Biol. 1995;172:126–138. - PubMed
- Bixby S, Kruger GM, Mosher JT, Joseph NM, Morrison SJ. Cell-intrinsic differences between stem cells from different regions of the peripheral nervous system regulate the generation of neural diversity. Neuron. 2002;35:643–656. - PubMed
- Bozzola JJ, Russell LD. Electron microscopy: principles and techniques for biologists. Boston, MA: Jones & Bartlett Publishers; 1992.
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS040750/NS/NINDS NIH HHS/United States
- P60-DK20572/DK/NIDDK NIH HHS/United States
- P30 CA046592/CA/NCI NIH HHS/United States
- AR20557/AR/NIAMS NIH HHS/United States
- R01 NS40750/NS/NINDS NIH HHS/United States
- CA46592/CA/NCI NIH HHS/United States
- P60 DK020572/DK/NIDDK NIH HHS/United States
- P30 AR048310/AR/NIAMS NIH HHS/United States
- 1 P30 AR48310/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical