Anillin binds nonmuscle myosin II and regulates the contractile ring - PubMed (original) (raw)
Anillin binds nonmuscle myosin II and regulates the contractile ring
Aaron F Straight et al. Mol Biol Cell. 2005 Jan.
Abstract
We demonstrate that the contractile ring protein anillin interacts directly with nonmuscle myosin II and that this interaction is regulated by myosin light chain phosphorylation. We show that despite their interaction, anillin and myosin II are independently targeted to the contractile ring. Depletion of anillin in Drosophila or human cultured cells results in cytokinesis failure. Human cells depleted for anillin fail to properly regulate contraction by myosin II late in cytokinesis and fail in abscission. We propose a role for anillin in spatially regulating the contractile activity of myosin II during cytokinesis.
Figures
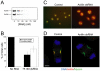
Figure 8.
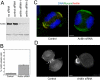
Depletion of anillin by siRNA in HeLa cells. (A) Western blot with anti-anillin of whole cell lysates after treatment with no RNA, control siRNA, or siRNA directed against human anillin. (B) Frequency of multinucleation in control or siRNA-treated HeLa cells. (C) immunofluorescence staining of DNA (blue), anillin (green), and myosin II (red) in HeLa cells treated with control or anillin siRNA. (D) Single frames of YFP-Myosin fluorescence from Movie S4, control, and Movie S8, anillin siRNA. White arrow indicates position of contractile ring. Bars, 5 μm.
Figure 1.
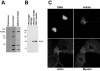
Characterization of X. laevis anillin. (A) Western blot of 20 μg of crude X. laevis egg extract with preimmune serum, immunized serum, and affinity-purified antibody. (B) Coimmunoprecipitation of anillin and actin from Xenopus egg extracts. Lane 1, interphase extract precipitated with rabbit IgG; lane 2, CSF extract precipitated with IgG; lane 3, interphase extract precipitated with anti-anillin; lane 4, CSF extract precipitated with anti-anillin. (C) immunofluorescence images XTC cells stained for DNA, anillin, actin, and myosin II.
Figure 2.
Anillin affinity chromatography. (A) Eluates from anillin (full length [FL] or without pleckstrin homology domain [-PH]), Polo kinase (PLX1), or GST columns. Left, cytostatic factor arrested extracts. Right, interphase extracts. (B) Eluates from GST and fulllength anillin columns immunoblotted with antibodies to nonmuscle myosin II heavy chain.
Figure 3.
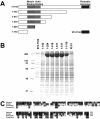
Delineation of myosin II binding region in anillin. (A) Schematic of anillin truncation fragments with minimal myosin binding region, region homologous to actin binding region, and pleckstrin homology domain highlighted. (B) Eluates from affinity columns for each of the fragments of anillin and Polo kinase (PLX1). Myosin II is the prominent band at 200 kDa. (C) Homology alignment of Xenopus, human, and Drosophila anillin in the myosin binding region.
Figure 4.
Anillin binds activated myosin II. Myosin II precipitation by anillin after myosin II phosphorylation by MLCK, Cdc2/CyclinB, Polo kinase, or no kinase.
Figure 5.
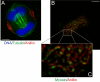
Myosin II and anillin organization in arrested contractile rings. (A) DNA, tubulin, and anillin staining in a blebbistatin-arrested HeLa cell. (B) Image plane at the coverslip surface in a blebbistatin-arrested HeLa cell stained for anillin and myosin II. (C) Enlarged view of B. Bars, 5 μm.
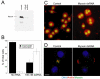
Figure 6.
Depletion of anillin by dsRNA in Drosophila cells. (A) Western blot of anillin depletion at varying dsRNA concentrations. (B) Quantitation of multinucleate phenotype in untreated or 75 nM anillin dsRNA-treated cells. (C) Image of control or anillin dsRNA-treated cells stained for DNA and cell volume. (D) Image of Drosophila cell untreated or anillin dsRNA treated during cytokinesis stained for DNA, anillin, and myosin II. Bar, 5 μm.
Figure 7.
Depletion of myosin II by dsRNA in Drosophila cells. (A) Western blot of myosin II after control and 100 nM myosin II dsRNA treatment. (B) Quantitation of multinucleate phenotype in untreated or myosin II dsRNA-treated cells. (C) DNA staining of multinucleate cells in control or myosin II dsRNA-treated cells. (D) Image of control or myosin II dsRNA-treated cells during cytokinesis stained for DNA, anillin, and myosin II. Bar, 5 μm.
Similar articles
- Stabilization of the actomyosin ring enables spermatocyte cytokinesis in Drosophila.
Goldbach P, Wong R, Beise N, Sarpal R, Trimble WS, Brill JA. Goldbach P, et al. Mol Biol Cell. 2010 May 1;21(9):1482-93. doi: 10.1091/mbc.e09-08-0714. Epub 2010 Mar 17. Mol Biol Cell. 2010. PMID: 20237160 Free PMC article. - Early zygotic gene product Dunk interacts with anillin to regulate Myosin II during Drosophila cleavage.
Chen J, Verissimo AF, Kull AR, He B. Chen J, et al. Mol Biol Cell. 2023 Sep 1;34(10):ar102. doi: 10.1091/mbc.E22-02-0046. Epub 2023 Jul 26. Mol Biol Cell. 2023. PMID: 37494082 Free PMC article. - Supervillin binding to myosin II and synergism with anillin are required for cytokinesis.
Smith TC, Fridy PC, Li Y, Basil S, Arjun S, Friesen RM, Leszyk J, Chait BT, Rout MP, Luna EJ. Smith TC, et al. Mol Biol Cell. 2013 Dec;24(23):3603-19. doi: 10.1091/mbc.E12-10-0714. Epub 2013 Oct 2. Mol Biol Cell. 2013. PMID: 24088567 Free PMC article. - How to scaffold the contractile ring for a safe cytokinesis - lessons from Anillin-related proteins.
D'Avino PP. D'Avino PP. J Cell Sci. 2009 Apr 15;122(Pt 8):1071-9. doi: 10.1242/jcs.034785. J Cell Sci. 2009. PMID: 19339546 Review. - Anillin: a pivotal organizer of the cytokinetic machinery.
Hickson GR, O'Farrell PH. Hickson GR, et al. Biochem Soc Trans. 2008 Jun;36(Pt 3):439-41. doi: 10.1042/BST0360439. Biochem Soc Trans. 2008. PMID: 18481976 Free PMC article. Review.
Cited by
- Tumor treating fields perturb the localization of septins and cause aberrant mitotic exit.
Gera N, Yang A, Holtzman TS, Lee SX, Wong ET, Swanson KD. Gera N, et al. PLoS One. 2015 May 26;10(5):e0125269. doi: 10.1371/journal.pone.0125269. eCollection 2015. PLoS One. 2015. PMID: 26010837 Free PMC article. - Drosophila melanogaster as a Model System for Human Glioblastomas.
Chen AS, Read RD. Chen AS, et al. Adv Exp Med Biol. 2019;1167:207-224. doi: 10.1007/978-3-030-23629-8_12. Adv Exp Med Biol. 2019. PMID: 31520357 Review. - Chromosomes function as a barrier to mitotic spindle bipolarity in polyploid cells.
Goupil A, Nano M, Letort G, Gemble S, Edwards F, Goundiam O, Gogendeau D, Pennetier C, Basto R. Goupil A, et al. J Cell Biol. 2020 Apr 6;219(4):e201908006. doi: 10.1083/jcb.201908006. J Cell Biol. 2020. PMID: 32328633 Free PMC article. - CLIC4 is a cytokinetic cleavage furrow protein that regulates cortical cytoskeleton stability during cell division.
Peterman E, Valius M, Prekeris R. Peterman E, et al. J Cell Sci. 2020 May 14;133(9):jcs241117. doi: 10.1242/jcs.241117. J Cell Sci. 2020. PMID: 32184265 Free PMC article. - A theoretical model of cytokinesis implicates feedback between membrane curvature and cytoskeletal organization in asymmetric cytokinetic furrowing.
Dorn JF, Zhang L, Phi TT, Lacroix B, Maddox PS, Liu J, Maddox AS. Dorn JF, et al. Mol Biol Cell. 2016 Apr 15;27(8):1286-99. doi: 10.1091/mbc.E15-06-0374. Epub 2016 Feb 24. Mol Biol Cell. 2016. PMID: 26912796 Free PMC article.
References
- Amano, M., Ito, M., Kimura, K., Fukata, Y., Chihara, K., Nakano, T., Matsuura, Y., and Kaibuchi, K. (1996). Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J. Biol. Chem. 271, 20246-20249. - PubMed
- Bhatia-Dey, N., Taira, M., Conti, M. A., Nooruddin, H., and Adelstein, R. S. (1998). Differential expression of non-muscle myosin heavy chain genes during Xenopus embryogenesis. Mech. Dev. 78, 33-36. - PubMed
- Daniel, J. L., and Sellers, J. R. (1992). Purification and characterization of platelet myosin. Methods Enzymol. 215, 78-88. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases