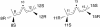A single active site residue directs oxygenation stereospecificity in lipoxygenases: stereocontrol is linked to the position of oxygenation - PubMed (original) (raw)
A single active site residue directs oxygenation stereospecificity in lipoxygenases: stereocontrol is linked to the position of oxygenation
Gianguido Coffa et al. Proc Natl Acad Sci U S A. 2004.
Abstract
Lipoxygenases are a class of dioxygenases that form hydroperoxy fatty acids with distinct positional and stereo configurations. Several amino acid residues influencing regiospecificity have been identified, whereas the basis of stereocontrol is not understood. We have now identified a single residue in the lipoxygenase catalytic domain that is important for stereocontrol; it is conserved as an Ala in S lipoxygenases and a Gly in R lipoxygenases. Our results with mutation of the conserved Ala to Gly in two S lipoxygenases (mouse 8S-LOX and human 15-LOX-2) and the corresponding Gly-Ala substitution in two R lipoxygenases (human 12R-LOX and coral 8R-LOX) reveal that the basis for R or S stereo-control also involves a switch in the position of oxygenation on the substrate. After the initial hydrogen abstraction, antarafacial oxygenation at one end or the other of the activated pair of double bonds (pentadiene) gives, for example, 8S or 12R product. The Ala residue promotes oxygenation on the reactive pentadiene at the end deep in the substrate binding pocket and S stereochemistry of the product hydroperoxide, and a Gly residue promotes oxygenation at the proximal end of the reactive pentadiene resulting in R stereochemistry. A model of lipoxygenase reaction specificity is proposed in which product regiochemistry and stereochemistry are determined by fixed relationships between substrate orientation, hydrogen abstraction, and the Gly or Ala residue we have identified.
Figures
Fig. 1.
Alignment of R and S lipoxygenases: identification of a potential stereodeterminant. A representative selection of S lipoxygenase enzymes are aligned with all of the R lipoxygenases of known primary structure. The highlighted residue is conserved as an Ala in all of the S lipoxygenases except for the mouse platelet 12_S_-LOX, where it is replaced by Ser. The equivalent residue in R lipoxygenase is conserved as Gly. Ep 12_S_-LOX, epidermal-type 12-LOX; leu 12_S_-LOX, leukocyte-type 12-LOX; pl 12_S_-LOX, platelet-type 12-LOX; coral 8_R_-LOX domain, the lipoxygenase domain of the peroxidase-lipoxygenase fusion protein of Plexaura homomalla (26).
Fig. 2.
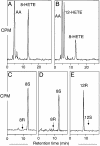
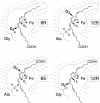
SP-HPLC and chiral HPLC analysis of products formed by WT and mutant mouse 8_S_-LOX. (A) SP-HPLC analysis for WT. (B) SP-HPLC analysis for Ala417Gly mutant. (C) Chiral HPLC analysis of 8-HETE-Me (8-HETE methyl ester) from WT. (D) Chiral HPLC analysis of 8-HETE-Me from Ala417Gly mutant. (E) Chiral HPLC analysis for 12-HETE-Me from Ala417Gly mutant. [1-14C]AA (100 μM) was incubated with equal amounts of HeLa cell homogenates expressing WT and mutant mouse 8_S_-LOX as described under Experimental Procedures. Products were analyzed by using a Whatman Partisil 5 silica column (0.46 × 25 cm) eluted at a flow rate of 1 ml/min with hexane/isopropyl alcohol/acetic acid (100:2:0.1, by volume). Individual metabolites purified by both RP-HPLC and SP-HPLC were analyzed on a Daicel Chiralpak AD (0.46 × 25 cm) eluted at a flow rate of 1 ml/min with hexane/methanol (100:2, by volume) (25).
Fig. 3.
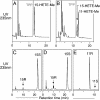
Metabolism of C16/AA-PC substrate by WT and mutant 15-LOX-2. (A) RP-HPLC analysis of WT. (B) RP-HPLC analysis of Ala416Gly mutant. (C) Chiral HPLC of 15-HETE-Me from WT. (D) Chiral HPLC of 15-HETE-Me from Ala416Gly mutant. (E) Chiral HPLC of 11-HETE-Me from Ala416Gly mutant. Products from the incubation of C16/AA-PC (100 μM) with WT and Ala416Gly 15-LOX-2 were reduced with TPP, transesterified to the methyl esters, and analyzed on a Waters Symmetry C18 5-μm column by using a solvent of methanol/water/acetic acid (85:15:0.01, by volume) and eluting at 1 ml/min with UV detection at 235 nm. Chiral HPLC analysis was carried out as described in the legend to Fig. 2.
Fig. 4.
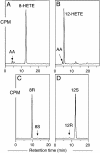
SP-HPLC and chiral HPLC analysis of products formed by WT and mutant coral 8_R_-LOX. (A) SP-HPLC analysis of WT. (B) SP-HPLC analysis of Gly427Ala mutant. (C) Chiral HPLC analysis of 8-HETE-Me from WT. (D) Chiral HPLC analysis of 12-HETE-Me from Gly427Ala mutant. [1-14C]AA (100 μM) was incubated with aliquots of WT and mutant coral 8_R_-LOX as described in Experimental Procedures. SP-HPLC and chiral HPLC analyses were carried out as described in the legend to Fig. 2.
Fig. 5.
Relationship between R and S stereospecificity in lipoxygenases. Reaction is illustrated at the 8,11 (Left) and 11,14 (Right) double bonds of a polyunsaturated fatty acid substrate such as AA. In a lipoxygenase-catalyzed reaction, after the initial hydrogen abstraction, oxygen can react on the opposite face of the substrate at one or the other end of the pentadiene radical. Thus, there is a relationship between 8_S_/12_R_ and 8_R_/12_S_ (Left) and, in the same way, between 11_R_/15_S_ and 11_S_/15_R_ (Right).
Fig. 6.
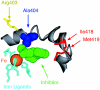
Location of the conserved Ala-404 residue in the active site of rabbit reticulocyte 15-LOX-1. In this detailed view from the crystal structure (29), iron (orange) in the center of the catalytic domain is held in place by conserved ligands (light blue). The sixth coordination position faces an open cavity, occupied here by a lipoxygenase inhibitor (green) and is presumably the location of fatty acid during catalysis. The conserved Ala-404 (blue) lies beyond the iron in a suitable position for influencing oxygenation of the activated substrate. On the far side of the protein, there is visible access into the active site over the polypeptide chain of Ala-404. Residues on the lower-right side include Ile-418 and Met-419 (red), shown earlier to influence binding of the ω chain of substrate AA (14). On the top left is Arg-403 (yellow), a residue exposed on the far side of the protein surface and suggested to interact with the carboxylic end of AA in this enzyme (32).
Fig. 7.
A basis for R or S stereospecificity in the lipoxygenase active site. Formation of four different products is represented in lipoxygenase active sites of related structure. In 8_R_-LOX (Upper Left) and 12_S_-LOX (Upper Right), AA has the same tail-first orientation in the active site, and reaction is initiated with the same
l
-hydrogen abstraction from one face of the substrate molecule. A Gly residue in the critical position in the proximal area of the active site allows antarafacial oxygenation at the proximal end of the reactive pentadiene in the 8_R_ configuration (Upper Left). Substitution with the larger Ala residue prevents oxygen insertion at C-8 and, instead, the antarafacial oxygenation occurs deep in the binding pocket in the 12_S_ configuration (Upper Right). In 8_S_-LOX (Lower Left) and 12_R_-LOX (Lower Right), the substrate binds in the reverse orientation with the carboxyl end in the active site, allowing removal of the
d
-hydrogen from C-10. A Gly residue allows oxygenation at the proximal end of the reacting substrate in the 12_R_ configuration (Lower Right), whereas the larger Ala residue prevents this, and the antarafacial oxygenation occurs deeper in the active site in the 8_S_ configuration (Lower Left). The CH2 hydrogens are labeled as
d
or
l
, because unlike the corresponding pro-R or pro-S designations, the Fischer nomenclature has a fixed right or left connotation all along the carbon chain (33).
Similar articles
- A comprehensive model of positional and stereo control in lipoxygenases.
Coffa G, Schneider C, Brash AR. Coffa G, et al. Biochem Biophys Res Commun. 2005 Dec 9;338(1):87-92. doi: 10.1016/j.bbrc.2005.07.185. Epub 2005 Aug 10. Biochem Biophys Res Commun. 2005. PMID: 16111652 Review. - Catalytic convergence of manganese and iron lipoxygenases by replacement of a single amino acid.
Wennman A, Jernerén F, Hamberg M, Oliw EH. Wennman A, et al. J Biol Chem. 2012 Sep 14;287(38):31757-65. doi: 10.1074/jbc.M112.364331. Epub 2012 Jul 20. J Biol Chem. 2012. PMID: 22822060 Free PMC article. - Insights from the X-ray crystal structure of coral 8R-lipoxygenase: calcium activation via a C2-like domain and a structural basis of product chirality.
Oldham ML, Brash AR, Newcomer ME. Oldham ML, et al. J Biol Chem. 2005 Nov 25;280(47):39545-52. doi: 10.1074/jbc.M506675200. Epub 2005 Sep 14. J Biol Chem. 2005. PMID: 16162493 - A G316A mutation of manganese lipoxygenase augments hydroperoxide isomerase activity: mechanism of biosynthesis of epoxyalcohols.
Cristea M, Oliw EH. Cristea M, et al. J Biol Chem. 2006 Jun 30;281(26):17612-23. doi: 10.1074/jbc.M510311200. Epub 2006 Apr 26. J Biol Chem. 2006. PMID: 16641090 - Lipoxygenase-catalyzed formation of R-configuration hydroperoxides.
Schneider C, Brash AR. Schneider C, et al. Prostaglandins Other Lipid Mediat. 2002 Aug;68-69:291-301. doi: 10.1016/s0090-6980(02)00041-2. Prostaglandins Other Lipid Mediat. 2002. PMID: 12432924 Review.
Cited by
- Manganese lipoxygenase of F. oxysporum and the structural basis for biosynthesis of distinct 11-hydroperoxy stereoisomers.
Wennman A, Magnuson A, Hamberg M, Oliw EH. Wennman A, et al. J Lipid Res. 2015 Aug;56(8):1606-15. doi: 10.1194/jlr.M060178. Epub 2015 Jun 25. J Lipid Res. 2015. PMID: 26113537 Free PMC article. - 12-Hydroxyeicosatetraenoic acid is the only enzymatically produced HETE increased under brain ischemia.
Golovko MY, Seeger DR, Schofield B, Besch D, Kotha P, Mansouripour A, Solaymani-Mohammadi S, Golovko SA. Golovko MY, et al. Prostaglandins Leukot Essent Fatty Acids. 2024 Mar;202:102631. doi: 10.1016/j.plefa.2024.102631. Epub 2024 Jul 19. Prostaglandins Leukot Essent Fatty Acids. 2024. PMID: 39059107 - Targeted knock-down of a structurally atypical zebrafish 12S-lipoxygenase leads to severe impairment of embryonic development.
Haas U, Raschperger E, Hamberg M, Samuelsson B, Tryggvason K, Haeggström JZ. Haas U, et al. Proc Natl Acad Sci U S A. 2011 Dec 20;108(51):20479-84. doi: 10.1073/pnas.1117094108. Epub 2011 Dec 5. Proc Natl Acad Sci U S A. 2011. PMID: 22143766 Free PMC article. - Structural and Functional Characterization of Lipoxygenases from Diatoms by Bioinformatics and Modelling Studies.
Giordano D, Bonora S, D'Orsi I, D'Alelio D, Facchiano A. Giordano D, et al. Biomolecules. 2024 Feb 25;14(3):276. doi: 10.3390/biom14030276. Biomolecules. 2024. PMID: 38540697 Free PMC article.
References
- Brash, A. R. (1999) J. Biol. Chem. 274, 23679-23682. - PubMed
- Kühn, H. & Thiele, B. J. (1999) FEBS Lett. 449, 7-11. - PubMed
- Funk, C. D. (2001) Science 294, 1871-1875. - PubMed
- Levy, B. D., De Sanctis, G. T., Devchand, P. R., Kim, E., Ackerman, K., Schmidt, B. A., Szczeklik, W., Drazen, J. M. & Serhan, C. N. (2002) Nat. Med. 8, 1018-1023. - PubMed
- Shureiqi, I. & Lippman, S. M. (2001) Cancer Res. 61, 6307-6312. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources