Molecular defects that affect platelet dense granules - PubMed (original) (raw)
Review
Molecular defects that affect platelet dense granules
Meral Gunay-Aygun et al. Semin Thromb Hemost. 2004 Oct.
Abstract
Platelet dense granules form using mechanisms shared by melanosomes in melanocytes and by subsets of lysosomes in more generalized cells. Consequently, disorders of platelet dense granules can reveal how organelles form and move within cells. Models for the study of new vesicle formation include isolated delta-storage pool deficiency, combined alphadelta-storage pool deficiency, Hermansky-Pudlak syndrome (HPS), Chediak-Higashi syndrome, Griscelli syndrome, thrombocytopenia absent radii syndrome, and Wiskott-Aldrich syndrome. The molecular bases of dense granule deficiency are known for the seven subtypes of HPS, as well as for Chediak-Higashi syndrome, Griscelli syndrome, and Wiskott-Aldrich syndrome. The gene products involved in these disorders help elucidate the generalized process of the formation of vesicles from extant membranes such as the Golgi.
Figures
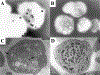
Figure 1
Electron microscopy of platelets showing presence and absence of dense bodies. (A) Whole-mount electron microscopy showing dark, electron-dense granules in the platelets of a 45-year-old woman with albinism but not Hermansky-Pudlak syndrome (HPS). (B) Whole-mount electron microscopy showing absence of dense bodies in the platelets of a 34-year-old man with HPS-1. (C) Transmission electron microscopy showing dense bodies (arrows) in the platelet of a patient with gray platelet syndrome. Note absence of α-granules. (D) Transmission electron microscopy showing absence of dense bodies in a platelet from a 30-year-old woman with HPS-1. Double arrow points to α-granules. (Photographs courtesy of Dr. James G. White, University of Minnesota.)
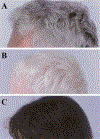
Figure 2
Hair of three HPS patients, all of Puerto Rican ancestry. (A) Typical gray-brown hair of a 54-year-old man with HPS-1. (B) Completely white hair of a 4-year-old boy with HPS-1. (C) Nearly normal, dark hair of a 5-year-old girl with HPS-3.
Figure 3
Intracellular distribution of LAMP3 and F-actin in normal, Hermansky-Pudlak syndrome (HPS)-1, HPS-3, and HPS-5 fibroblasts. Fibroblasts were fixed on coverslips and stained with mouse monoclonal antibodies to LAMP3 (as described in Huizing et al) and F-actin (Phalloidin, Molecular Probes, Eugene, OR). F-actin staining (red) was employed to mark the outline of the cells, in particular the dendritic tips. Insets show isolated dendritic tips. (A) Normal fibroblasts show a punctate LAMP3 (green) distribution throughout the cell, including at the ends of the dendritic tips. (B) HPS-1 fibroblasts show a clumped accumulation of LAMP3 in the perinuclear area. (C) HPS-3 fibroblasts appear to have a nearly normal LAMP3 distribution, with LAMP3 reaching into the dendritic tips. (D) HPS-5 cells have minimal LAMP3 transport into the tips, but LAMP3 does not accumulate in clumps in the perinuclear area, as in HPS-1. (Photographs produced by Heidi Dorward.)
Similar articles
- Hermansky-Pudlak syndrome and Chediak-Higashi syndrome: disorders of vesicle formation and trafficking.
Huizing M, Anikster Y, Gahl WA. Huizing M, et al. Thromb Haemost. 2001 Jul;86(1):233-45. Thromb Haemost. 2001. PMID: 11487012 Review. - Electron-dense chains and clusters in platelets from patients with storage pool-deficiency disorders.
White JG. White JG. J Thromb Haemost. 2003 Jan;1(1):74-9. doi: 10.1046/j.1538-7836.2003.00032.x. J Thromb Haemost. 2003. PMID: 12871542 - The regulation of platelet-dense granules by Rab27a in the ashen mouse, a model of Hermansky-Pudlak and Griscelli syndromes, is granule-specific and dependent on genetic background.
Novak EK, Gautam R, Reddington M, Collinson LM, Copeland NG, Jenkins NA, McGarry MP, Swank RT. Novak EK, et al. Blood. 2002 Jul 1;100(1):128-35. doi: 10.1182/blood.v100.1.128. Blood. 2002. PMID: 12070017 - Electron opaque structures in human platelets: which are or are not dense bodies?
White JG. White JG. Platelets. 2008 Sep;19(6):455-66. doi: 10.1080/09537100802132671. Platelets. 2008. PMID: 18925514 - Platelet granule disorders.
White JG. White JG. Crit Rev Oncol Hematol. 1986;4(4):337-77. doi: 10.1016/s1040-8428(86)80027-0. Crit Rev Oncol Hematol. 1986. PMID: 3513985 Review.
Cited by
- Role of MRP4 (ABCC4) in platelet adenine nucleotide-storage: evidence from patients with delta-storage pool deficiencies.
Jedlitschky G, Cattaneo M, Lubenow LE, Rosskopf D, Lecchi A, Artoni A, Motta G, Niessen J, Kroemer HK, Greinacher A. Jedlitschky G, et al. Am J Pathol. 2010 Mar;176(3):1097-103. doi: 10.2353/ajpath.2010.090425. Epub 2010 Feb 4. Am J Pathol. 2010. PMID: 20133816 Free PMC article. - Genetic classification and confirmation of inherited platelet disorders: current status in Korea.
Shim YJ. Shim YJ. Clin Exp Pediatr. 2020 Mar;63(3):79-87. doi: 10.3345/kjp.2019.00052. Epub 2020 Feb 6. Clin Exp Pediatr. 2020. PMID: 31477680 Free PMC article. - Disorders of lysosome-related organelle biogenesis: clinical and molecular genetics.
Huizing M, Helip-Wooley A, Westbroek W, Gunay-Aygun M, Gahl WA. Huizing M, et al. Annu Rev Genomics Hum Genet. 2008;9:359-86. doi: 10.1146/annurev.genom.9.081307.164303. Annu Rev Genomics Hum Genet. 2008. PMID: 18544035 Free PMC article. Review. - Masks of Albinism: Clinical Spectrum of Hermansky-Pudlak Syndrome.
Bobreshova AM, Ionova SA, Kadyshev VV, Sukhanova NV, Viakhireva IV, Filatova AY, Zhurkova NV, Sparber PA, Marakhonov AV, Vasilyeva TA, Shchagina OA, Kutsev SI, Zinchenko RA. Bobreshova AM, et al. Int J Mol Sci. 2024 Oct 19;25(20):11260. doi: 10.3390/ijms252011260. Int J Mol Sci. 2024. PMID: 39457042 Free PMC article. - Serum antibody-negative Goodpasture syndrome with delta granule pool storage deficiency and eosinophilia.
Kussman A, Gohara A. Kussman A, et al. Clin Kidney J. 2012 Dec;5(6):572-5. doi: 10.1093/ckj/sfs107. Epub 2012 Oct 19. Clin Kidney J. 2012. PMID: 26069804 Free PMC article.
References
- Israels SJ, McNicol A, Robertson C, Gerrard GM. Platelet storage pool deficiency: diagnosis in patients with prolonged bleeding times and normal platelet aggregation. Br J Haematol 1990;75:118–121 - PubMed
- Witkop CJ, Krumwiede M, Sedano H, White JG. Reliability of absent platelet dense bodies as a diagnostic criterion for Hermansky-Pudlak syndrome. Am J Hematol 1987;26:305–311 - PubMed
- McNicol A, Israels S. Platelet dense granules: structure, function and implications for haemostasis. Thromb Res 1999; 95:1–18 - PubMed
- Holmsen H, Weiss HJ. Secretable storage pools in platelets. Annu Rev Med 1979;30:119–134 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical