A cluster of metabolic defects caused by mutation in a mitochondrial tRNA - PubMed (original) (raw)
. 2004 Nov 12;306(5699):1190-4.
doi: 10.1126/science.1102521. Epub 2004 Oct 21.
Ali Hariri, Anita Farhi, Hongyu Zhao, Kitt Falk Petersen, Hakan R Toka, Carol Nelson-Williams, Khalid M Raja, Michael Kashgarian, Gerald I Shulman, Steven J Scheinman, Richard P Lifton
Affiliations
- PMID: 15498972
- PMCID: PMC3033655
- DOI: 10.1126/science.1102521
A cluster of metabolic defects caused by mutation in a mitochondrial tRNA
Frederick H Wilson et al. Science. 2004.
Abstract
Hypertension and dyslipidemia are risk factors for atherosclerosis and occur together more often than expected by chance. Although this clustering suggests shared causation, unifying factors remain unknown. We describe a large kindred with a syndrome including hypertension, hypercholesterolemia, and hypomagnesemia. Each phenotype is transmitted on the maternal lineage with a pattern indicating mitochondrial inheritance. Analysis of the mitochondrial genome of the maternal lineage identified a homoplasmic mutation substituting cytidine for uridine immediately 5' to the mitochondrial transfer RNA(Ile) anticodon. Uridine at this position is nearly invariate among transfer RNAs because of its role in stabilizing the anticodon loop. Given the known loss of mitochondrial function with aging, these findings may have implications for the common clustering of these metabolic disorders.
Figures
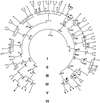
Fig. 1
The structure of Kindred 129. Individuals with serum Mg2+ < 1.8 mg/dl are indicated by black symbols. Family members taking antihypertensive medications or having blood pressures over 140/90 mm Hg are indicated by an H. Members with hypercholesterolemia (serum cholesterol > 200 mg/dl or taking lipid-lowering agents) are denoted by C. Blood relatives who did not have electrolyte values measured are indicated by gray symbols. The index case is indicated by an arrow.
Fig. 2
Renal hypomagnesemia, hypocalciuria, and hypokalemia in the maternal lineage of K129. (A) Serum Mg2+ values for individuals in maternal and nonmaternal lineages of K129 are shown and are significantly different (P = 2 × 10−9). (B) Fractional renal Mg2+ excretion (FEMg2+) on the maternal and nonmaternal lineages is shown; on the maternal lineage, individuals with normal and low Mg2+ levels are separated. Hypomagnesemic subjects have significantly elevated fractional excretion of Mg2+, indicating a renal defect (P = 0.0001 comparing maternal to nonmaternal; P = 5 × 10−6 comparing hypomagnesemic subjects versus those not in the maternal lineage). (C) Urinary calcium to creatinine ratios (UCa/cr) are shown grouped as in (B); maternal subjects have significantly reduced urinary calcium levels (P = 0.0005). (D) Serum K+ levels. Hypokalemia is seen predominantly on the maternal lineage among hypomagnesemic subjects.
Fig. 3
Quantitative blood pressure and cholesterol values in K129. Values in maternal and nonmaternal K129 members between the ages of 18 and 60 are shown. All values represent difference from the mean value after adjustment for age, sex, and BMI. For blood pressure and lipids, units are mm Hg and mg/dl, respectively. Mean and SEM values are indicated for maternal and nonmaternal groups. Values are significantly elevated in members of the maternal lineage. (A) Systolic blood pressure (P = 0.00007). (B) Diastolic blood pressure (P = 0.002). (C) Fasting total cholesterol (P = 0.002). (D) Fasting LDL + VLDL cholesterol (P = 0.004).
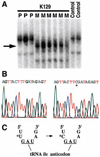
Fig. 4
Mitochondrial tRNAIle mutation in K129. Mitochondrial DNA from both blood leukocytes and renal epithelial cells was analyzed and yielded identical results. (A) A fragment of mtDNA containing the tRNAIle gene was amplified from members of K129 and normal controls and was fractionated by nondenaturing gel electrophoresis (18). A thymidine-to-cytidine variant (indicated by arrow) is present in individuals from the maternal lineage (M) but absent in offspring of affected males (paternal lineage, P) and unrelated controls. (B) The sequence of a portion of the mitochondrial tRNAIle gene from the amplicon in (A) from a wild-type control (left) and a member of the maternal lineage of K129 (right). A single base substitution (asterisk) changes the wild-type thymidine to cytidine. (C) The T → C transition alters the nucleotide immediately 5′ to the tRNAIle anticodon.
Similar articles
- A homoplasmic mitochondrial transfer ribonucleic acid mutation as a cause of maternally inherited hypertrophic cardiomyopathy.
Taylor RW, Giordano C, Davidson MM, d'Amati G, Bain H, Hayes CM, Leonard H, Barron MJ, Casali C, Santorelli FM, Hirano M, Lightowlers RN, DiMauro S, Turnbull DM. Taylor RW, et al. J Am Coll Cardiol. 2003 May 21;41(10):1786-96. doi: 10.1016/s0735-1097(03)00300-0. J Am Coll Cardiol. 2003. PMID: 12767666 - Medicine. Metabolic defects tied to mitochondrial gene.
Marx J. Marx J. Science. 2004 Oct 22;306(5696):592-3. doi: 10.1126/science.306.5696.592b. Science. 2004. PMID: 15498983 No abstract available. - [Search for mitochondrial DNA T4291C mutation in Hungarian patients with metabolic syndrome].
Maász A, Horvatovich K, Magyari L, Talián Csaba G, Bokor S, Laczy B, Tamaskó M, Molnár D, Wittmann I, Melegh B. Maász A, et al. Orv Hetil. 2006 Apr 16;147(15):693-6. Orv Hetil. 2006. PMID: 16734181 Hungarian. - Enzymatic conversion of cytidine to lysidine in anticodon of bacterial isoleucyl-tRNA--an alternative way of RNA editing.
Grosjean H, Björk GR. Grosjean H, et al. Trends Biochem Sci. 2004 Apr;29(4):165-8. doi: 10.1016/j.tibs.2004.02.009. Trends Biochem Sci. 2004. PMID: 15124629 Review. No abstract available. - Mutations of Nuclear and Mitochondrial Genomes as Potential Targets for the Treatment of Metabolic Syndrome.
Galitsyna EV, Zhelankin AV, Sobenin IA, Orekhov AN. Galitsyna EV, et al. Curr Pharm Des. 2018;24(15):1711-1716. doi: 10.2174/1381612824666180115120725. Curr Pharm Des. 2018. PMID: 29336249 Review.
Cited by
- Polycystic ovary syndrome and mitochondrial dysfunction.
Zhang J, Bao Y, Zhou X, Zheng L. Zhang J, et al. Reprod Biol Endocrinol. 2019 Aug 16;17(1):67. doi: 10.1186/s12958-019-0509-4. Reprod Biol Endocrinol. 2019. PMID: 31420039 Free PMC article. Review. - Therapeutic promise and principles: metabotropic glutamate receptors.
Maiese K, Chong ZZ, Shang YC, Hou J. Maiese K, et al. Oxid Med Cell Longev. 2008 Oct-Dec;1(1):1-14. doi: 10.4161/oxim.1.1.6842. Oxid Med Cell Longev. 2008. PMID: 19750024 Free PMC article. Review. - Genetic association analysis of 13 nuclear-encoded mitochondrial candidate genes with type II diabetes mellitus: the DAMAGE study.
Reiling E, van Vliet-Ostaptchouk JV, van 't Riet E, van Haeften TW, Arp PA, Hansen T, Kremer D, Groenewoud MJ, van Hove EC, Romijn JA, Smit JW, Nijpels G, Heine RJ, Uitterlinden AG, Pedersen O, Slagboom PE, Maassen JA, Hofker MH, 't Hart LM, Dekker JM. Reiling E, et al. Eur J Hum Genet. 2009 Aug;17(8):1056-62. doi: 10.1038/ejhg.2009.4. Epub 2009 Feb 11. Eur J Hum Genet. 2009. PMID: 19209188 Free PMC article. - Increased protein nitration in mitochondrial diseases: evidence for vessel wall involvement.
Vattemi G, Mechref Y, Marini M, Tonin P, Minuz P, Grigoli L, Guglielmi V, Klouckova I, Chiamulera C, Meneguzzi A, Di Chio M, Tedesco V, Lovato L, Degan M, Arcaro G, Lechi A, Novotny MV, Tomelleri G. Vattemi G, et al. Mol Cell Proteomics. 2011 Apr;10(4):M110.002964. doi: 10.1074/mcp.M110.002964. Epub 2010 Dec 14. Mol Cell Proteomics. 2011. PMID: 21156839 Free PMC article. - Evaluation of genetic variation contributing to differences in gene expression between populations.
Zhang W, Duan S, Kistner EO, Bleibel WK, Huang RS, Clark TA, Chen TX, Schweitzer AC, Blume JE, Cox NJ, Dolan ME. Zhang W, et al. Am J Hum Genet. 2008 Mar;82(3):631-40. doi: 10.1016/j.ajhg.2007.12.015. Epub 2008 Feb 28. Am J Hum Genet. 2008. PMID: 18313023 Free PMC article.
References
- Stamler J, Wentworth D, Neaton JD. JAMA. 1986;256:2823. - PubMed
- Mosterd A, et al. N. Engl. J. Med. 1999;340:1221. - PubMed
- Wingard DL, Barrett-Connor E, Criqui MH, Suarez L. Am. J. Epidemiol. 1983;117:19. - PubMed
- Reaven GM. Diabetes. 1988;37:1595. - PubMed
- Criqui MH, et al. Circulation. 1986;73:I40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 HL055007/HL/NHLBI NIH HHS/United States
- R01 DK049230/DK/NIDDK NIH HHS/United States
- R01 AG023686-04/AG/NIA NIH HHS/United States
- R01 AG023686-01A1/AG/NIA NIH HHS/United States
- R01 AG023686/AG/NIA NIH HHS/United States
- R01 AG023686-02/AG/NIA NIH HHS/United States
- MO1 RR-00125/RR/NCRR NIH HHS/United States
- R01 DK-49230/DK/NIDDK NIH HHS/United States
- R01 AG023686-03/AG/NIA NIH HHS/United States
- M01 RR000125/RR/NCRR NIH HHS/United States
- P50 HL-55007/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases