Functional characterization of two type III secretion systems of Vibrio parahaemolyticus - PubMed (original) (raw)
Functional characterization of two type III secretion systems of Vibrio parahaemolyticus
Kwon-Sam Park et al. Infect Immun. 2004 Nov.
Abstract
Vibrio parahaemolyticus, a gram-negative marine bacterium, is a worldwide cause of food-borne gastroenteritis. Recent genome sequencing of the clinical V. parahaemolyticus strain RIMD2210633 identified two sets of genes for the type III secretion system (TTSS), TTSS1 and TTSS2. Here, we constructed a series of mutant strains from RIMD2210633 to determine whether the two putative TTSS apparatus are functional. The cytotoxic activity of mutant strains having a deletion in one of the TTSS1 genes was significantly decreased compared with that of the parent and TTSS2-related mutant strains. In an enterotoxicity assay with the rabbit ileal loop test, intestinal fluid accumulation was diminished by deletion of the TTSS2-related genes while TTSS1-related mutants caused a level of fluid accumulation similar to that of the parent. VopD, a protein encoded in the proximity of the TTSS1 region and a homologue of the Yersinia YopD, was secreted in a TTSS1-dependent manner. In contrast, VopP, which is encoded by a pathogenicity island on chromosome 2 and is homologous to the Yersinia YopP, was secreted via the TTSS2 pathway. These results provide evidence that V. parahaemolyticus TTSSs function as secretion systems and may have a role in the pathogenicity of the organism. This is the first report of functional TTSSs in Vibrio species. The presence of TTSS apparatus gene homologues was demonstrated in other vibrios, such as Vibrio alginolyticus, Vibrio harveyi, and Vibrio tubiashii, suggesting that some other vibrios also contain TTSS and that the TTSS has a role in protein secretion in those organisms during interaction with eukaryotic cells.
Figures
FIG. 1.
Organization of the V. parahaemolyticus TTSS1 locus. Numbers above the genes of V. parahaemolyticus TTSS1 are percent identities at the amino acid level to the corresponding proteins of Yersinia TTSS.
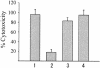
FIG. 2.
Cytotoxicity of parent and TTSS-related deletion mutant strains to HeLa cells. HeLa cells were infected with bacteria at a multiplicity of infection of 20. Five hours after infection, cytotoxic activity was assayed by measuring total cellular LDH release into the culture supernatant. The percent cytotoxicity was calculated by using the following equation: (OD490 of experimental release − OD490 of spontaneous release)/(OD490 of maximum release − OD490 of spontaneous release) × 100. Bars: 1, parent strain; 2, vcrD1 deletion mutant; 3, vcrD2 deletion mutant; 4, vcrD1 complementation into vcrD1 deletion mutant. Results are representative of at least three independent experiments.
FIG. 3.
Morphological changes induced by V. parahaemolyticus infection. HeLa cells were infected with the following strains: parent strain (A, B), vcrD1 deletion mutant (C, D), vcrD2 deletion mutant (E, F). Uninfected cells were used as a control (G, H). Five hours after infection, cells were fixed, stained, and observed by phase-contrast microscopy (left) and Hoechst staining (right).
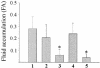
FIG. 4.
Fluid accumulation in the rabbit ileal loop test. Fluid accumulation (FA) is amount of accumulated fluid (in milliliters) per length (in centimeters) of ligated rabbit small intestine. Bars: 1, parent strain; 2, vcrD1 deletion mutant; 3, vcrD2 deletion mutant; 4, vcrD2 complementation into vcrD2 deletion mutant; 5, PBS. Data represented are means ± standard deviations. The fluid accumulation of 0.2 μg of cholera toxin (Sigma), used as a positive control, was 0.71 ± 0.12 ml/cm. Asterisks indicate significant differences from the results obtained with the parent strain (P < 0.01).
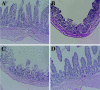
FIG. 5.
Hematoxylin and eosin staining of tissue from rabbit intestinal loops infected with 108 CFU of the parent strain (B), vcrD1 mutant (C), vcrD2 mutant (D), and PBS as a negative control (A) for 15 h. The parent and vcrD1 mutant strains showed blunting of villi, submucosal edema, hemorrhage, and severe inflammation.
FIG. 6.
Western blot analysis of proteins in culture supernatants of the parent and TTSS-related mutant strains grown in heart infusion broth medium for 5 h (for VopD) or in LB medium for 16 h (for VopP) at 37°C. Western blot analysis was performed by using the polyclonal antibodies against VopD (A) and VopP (B). Lane 1, parent strain (POR-1); lane 3, vcrD1 mutant; lane 4, vscC1 mutant; lane 5, vscN1 mutant; lane 6, vcrD2 mutant; lane 7, vscC2 mutant; lane 8, vscN2 mutant. Lane 2 in panel A is the vopD mutant, and lane 2 in panel B is the vopP mutant.
Similar articles
- Identification of proteins secreted via Vibrio parahaemolyticus type III secretion system 1.
Ono T, Park KS, Ueta M, Iida T, Honda T. Ono T, et al. Infect Immun. 2006 Feb;74(2):1032-42. doi: 10.1128/IAI.74.2.1032-1042.2006. Infect Immun. 2006. PMID: 16428750 Free PMC article. - Modulation of pathogenicity with norepinephrine related to the type III secretion system of Vibrio parahaemolyticus.
Nakano M, Takahashi A, Sakai Y, Nakaya Y. Nakano M, et al. J Infect Dis. 2007 May 1;195(9):1353-60. doi: 10.1086/513275. Epub 2007 Mar 15. J Infect Dis. 2007. PMID: 17397007 - [Type III secretion system of Vibrio parahaemolyticus--a review].
Yu Y, Wu B, Fang W. Yu Y, et al. Wei Sheng Wu Xue Bao. 2009 Jul;49(7):848-52. Wei Sheng Wu Xue Bao. 2009. PMID: 19873746 Review. Chinese. - The Vibrio parahaemolyticus Type III Secretion Systems manipulate host cell MAPK for critical steps in pathogenesis.
Matlawska-Wasowska K, Finn R, Mustel A, O'Byrne CP, Baird AW, Coffey ET, Boyd A. Matlawska-Wasowska K, et al. BMC Microbiol. 2010 Dec 30;10:329. doi: 10.1186/1471-2180-10-329. BMC Microbiol. 2010. PMID: 21192810 Free PMC article. - Advances on Vibrio parahaemolyticus research in the postgenomic era.
Matsuda S, Hiyoshi H, Tandhavanant S, Kodama T. Matsuda S, et al. Microbiol Immunol. 2020 Mar;64(3):167-181. doi: 10.1111/1348-0421.12767. Epub 2020 Jan 21. Microbiol Immunol. 2020. PMID: 31850542 Review.
Cited by
- Enteropathogenic Providencia alcalifaciens: A Subgroup of P. alcalifaciens That Causes Diarrhea.
Bulach D, Carter GP, Albert MJ. Bulach D, et al. Microorganisms. 2024 Jul 19;12(7):1479. doi: 10.3390/microorganisms12071479. Microorganisms. 2024. PMID: 39065247 Free PMC article. - Pathogenic Bacteria in Free-Living Birds, and Its Public Health Significance.
Kobuszewska A, Wysok B. Kobuszewska A, et al. Animals (Basel). 2024 Mar 20;14(6):968. doi: 10.3390/ani14060968. Animals (Basel). 2024. PMID: 38540066 Free PMC article. Review. - The read-through transcription-mediated autoactivation circuit for virulence regulator expression drives robust type III secretion system 2 expression in Vibrio parahaemolyticus.
Anggramukti DS, Ishii E, Pratama A, Al Kadi M, Iida T, Kodama T, Matsuda S. Anggramukti DS, et al. PLoS Pathog. 2024 Mar 27;20(3):e1012094. doi: 10.1371/journal.ppat.1012094. eCollection 2024 Mar. PLoS Pathog. 2024. PMID: 38536895 Free PMC article. - Unveiling potential virulence determinants in Vibrio isolates from Anadara tuberculosa through whole genome analyses.
Restrepo-Benavides M, Lozano-Arce D, Gonzalez-Garcia LN, Báez-Aguirre F, Ariza-Aranguren G, Faccini D, Zambrano MM, Jiménez P, Fernández-Bravo A, Restrepo S, Guevara-Suarez M. Restrepo-Benavides M, et al. Microbiol Spectr. 2024 Feb 6;12(2):e0292823. doi: 10.1128/spectrum.02928-23. Epub 2024 Jan 8. Microbiol Spectr. 2024. PMID: 38189292 Free PMC article. - ArcB orchestrates the quorum-sensing system to regulate type III secretion system 1 in Vibrio parahaemolyticus.
Zhang C, Liu M, Wu Y, Li X, Zhang C, Call DR, Liu M, Zhao Z. Zhang C, et al. Gut Microbes. 2023 Dec;15(2):2281016. doi: 10.1080/19490976.2023.2281016. Epub 2023 Nov 20. Gut Microbes. 2023. PMID: 37982663 Free PMC article.
References
- Aldridge, P., and K. T. Hughes. 2001. How and when are substrates selected for type III secretion? Trends Microbiol. 9:209-214. - PubMed
- Cornelis, G. R., and F. Van Gijsegem. 2000. Assembly and function of type III secretory systems. Annu. Rev. Microbiol. 54:735-774. - PubMed
- Daniels, N. A., L. MacKinnon, R. Bishop, S. Altekruse, et al. 2000. Vibrio parahaemolyticus infections in the United States, 1973-1998. J. Infect. Dis. 181:1661-1666. - PubMed
- Foultier, B., P. Troisfontaines, S. Muller, F. R. Opperdoes, and G. R. Cornelis. 2002. Characterization of the ysa pathogenicity locus in the chromosome of Yersinia enterocolitica and phylogeny analysis of type III secretion systems. J. Mol. Evol. 55:37-51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials