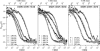Diffusion in two-component lipid membranes--a fluorescence correlation spectroscopy and monte carlo simulation study - PubMed (original) (raw)
Diffusion in two-component lipid membranes--a fluorescence correlation spectroscopy and monte carlo simulation study
Agnieszka E Hac et al. Biophys J. 2005 Jan.
Abstract
Using fluorescence correlation spectroscopy, calorimetry, and Monte Carlo simulations, we studied diffusion processes in two-component membranes close to the chain melting transition. The aim is to describe complex diffusion behavior in lipid systems in which gel and fluid domains coexist. Diffusion processes in gel membranes are significantly slower than in fluid membranes. Diffusion processes in mixed phase regions are therefore expected to be complex. Due to statistical fluctuations the gel-fluid domain patterns are not uniform in space and time. No models for such diffusion processes are available. In this article, which is both experimental and theoretical, we investigated the diffusion in DMPC-DSPC lipid mixtures as a function of temperature and composition. We then modeled the fluorescence correlation spectroscopy experiment using Monte Carlo simulations to analyze the diffusion process. It is shown that the simulations yield a very good description of the experimental diffusion processes, and that predicted autocorrelation profiles are superimposable with the experimental curves. We believe that this study adds to the discussion on the physical nature of rafts found in biomembranes.
Figures
FIGURE 1
(Left) Schematic drawing of the FCS setup and the simulations. The laser (green) is focused on planar membranes, which are predicted to contain domains. Fluorescence light from the focus (yellow) is projected on two avalanche photodiodes (APD) which monitor different polarizations. In the image plane a pinhole is located. (Center) Single molecule fluorescence intensity traces of rhodamine 6G in solution, TRITC in a fluid lipid membrane and TRITC in a gel lipid membrane. Note the different timescales. (Right) Autocorrelation of the fluorescence signals shown in the center panel. The diffusion timescale within the fluid membrane is much faster than in the gel membrane, but approximately two orders-of-magnitude slower than the free diffusion of a label in the bulk solvent. The solid lines represent fits according to Eq. 1.
FIGURE 2
(Left) State changes from gel to fluid of individual lipid chains in the Monte Carlo simulation. (Right) Possible nearest-neighbor exchange steps of lipids leading to diffusion. On the simulation matrix the lipid chains are located on a triangular lattice. All individual exchange steps were explored with the same frequency. The link between the two chains of a lipid is indicated by a bar.
FIGURE 3
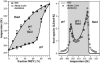
(Left) Phase-diagram, showing lower and upper limits of the calorimetric events. Regions of expected microscopic or macroscopic separation of gel and fluid regions are in shaded representation. (Right) Heat capacity profile of a DMPC:DSPC 50:50 mixture (solid line), and the corresponding Monte Carlo simulation (symbols).
FIGURE 4
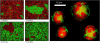
(Left) Representative Monte Carlo snapshots of a 50:50 DMPC:DSPC mixture at four temperatures below, within, and above the melting regime (see Fig. 3).The color code is shown in Fig. 2. (Red domains correspond to gel lipids, green domains to fluid lipids.) Note the different length scales of the domains (macroscopic and microscopic domains). (Right) Confocal microscopy image of a 30:70 DLPC:DPPC mixture at 306 K, showing domain formation (gel domains in red, fluid domains in green). Compare with the domain shapes in the simulation.
FIGURE 5
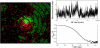
(Left) The fluorescence correlation experiment can be simulated in the Monte Carlo simulation by labeling some of the lipid chains with a marker (white dots) and by introduction of a focus with Gaussian cross section (concentric circles). (Upper right panel) Assuming instantaneous emission after excitation with a Gaussian detection probability, a fluorescence signal from the diffusing labeled lipids can be obtained. (Lower right panel) Autocorrelated profile of the signal in the upper panel.
FIGURE 6
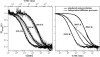
Experimental and theoretical autocorrelation functions of three DMPC/DSPC mixtures at different temperatures. (Left) 70:30 mixture at 289.3 K, 303.0 K, 309.2 K, and 319.2 K (below, within, and above the melting regime; see Fig. 3). (Center) 50:50 mixture at 290.5 K, 303.6 K, 309.7 K, and 322.5 K (below, within, and above the melting regime). (Right) 30:70 mixture at 291.0 K, 317.6 K, and 330.0 K (below, within, and above the melting regime). The experimental profiles are very well described by the simulation. It shall be noted that the simulation exclusively relies on the calorimetric input parameters and does not require any fitting, except for the adjustment of the timescales in the pure gel and the pure fluid phases. All intermediate profiles are predictions rather than fits.
FIGURE 7
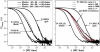
(Left) The experimental autocorrelation profiles are subject to some variation due to changes in location within the sample and due to slight temperature variations. Here, for three different temperatures of a 70:30 DMPC:DSPC mixture, the mean variation of the autocorrelation profiles is shown. For Fig. 6 representative profiles were chosen, which are given here as solid lines. The dotted lines are the predictions from simulations shown in Fig. 6. (Right) This panel shows that the correlation profiles from the two-phase regime are not well described by a superposition of a pure gel (right curves) and a pure fluid component (left curves) with properties identical to the pure phase (the ratios of the two components have been chosen according to Fig. 3 using the Lever rule). The dotted lines are superpositions of analytical profiles according to Eq. 1. The profiles from the Monte Carlo simulations (which are identical to the experiment) are given by the solid lines. See text for a discussion.
FIGURE 8
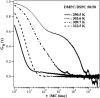
(Left) Dependence of the autocorrelation profile on increasing matrix and focal radius size: 60 × 60 with a focal radius of 25 chains, 80 × 80 with a focal radius of 38 chains, and 200 × 200 with a focal radius of 95 chains. The autocorrelation profiles are only slightly affected by the size of the simulation system. The 200 × 200 matrix corresponds to 100 nm × 83 nm, which is close to the experimental length scale. (Right) Dependence of the autocorrelation function on the relaxation timescale reflecting the frequency of attempts to change the state of a chain and to change position in the fluid state, indicated by a ratio of 1:1, 1:100, 1:1000, and 1:∞ (no state fluctuations). If the changes in state are less frequent than the positional changes, the slow components in the autocorrelation profile are more pronounced. This shows that the autocorrelation profile contains information about the relaxation timescales. The intermediate phase regime at all fluctuation rates could well be described by anomalous subdiffusion (red lines). Parameters are given in the text.
FIGURE 9
Autocorrelation of the enthalpy fluctuations of a DMPC:DSPC mixture from the simulations in Fig. 6 (center panel), indicating the lipid state relaxation times (Grabitz et al., 2002). The typical steps in the enthalpy fluctuations yield the lipid state relaxation time(s). The relaxation at the _c_P-maximum at 303.6 K is slowest, whereas it is fastest in the pure gel (290.5 K) and the fluid phases (322.5 K). Relaxation times at 303.6 K are between 101 and 105 Monte Carlo cycles, whereas the mean dwell-time of the label is ∼103 cycles. This indicates that relaxation processes may be slower than the time that a label spends in the microscope focus. For gel and fluid phase simulations (290.5 K and 322.5 K, respectively), relaxation is faster than the dwell-time of the label in the focus (see Fig. 6, center panel).
Similar articles
- Lateral diffusion of molecules in two-component lipid bilayer: a Monte Carlo simulation study.
Sugár IP, Biltonen RL. Sugár IP, et al. J Phys Chem B. 2005 Apr 21;109(15):7373-86. doi: 10.1021/jp045669x. J Phys Chem B. 2005. PMID: 16851844 - Melting of individual lipid components in binary lipid mixtures studied by FTIR spectroscopy, DSC and Monte Carlo simulations.
Fidorra M, Heimburg T, Seeger HM. Fidorra M, et al. Biochim Biophys Acta. 2009 Mar;1788(3):600-7. doi: 10.1016/j.bbamem.2008.12.003. Epub 2008 Dec 14. Biochim Biophys Acta. 2009. PMID: 19150329 - Topology of gel-phase domains and lipid mixing properties in phase-separated two-component phosphatidylcholine bilayers.
Schram V, Lin HN, Thompson TE. Schram V, et al. Biophys J. 1996 Oct;71(4):1811-22. doi: 10.1016/S0006-3495(96)79382-7. Biophys J. 1996. PMID: 8889158 Free PMC article. - Detecting nanodomains in living cell membrane by fluorescence correlation spectroscopy.
He HT, Marguet D. He HT, et al. Annu Rev Phys Chem. 2011;62:417-36. doi: 10.1146/annurev-physchem-032210-103402. Annu Rev Phys Chem. 2011. PMID: 21219145 Review. - Lipid domain formation and dynamics in giant unilamellar vesicles explored by fluorescence correlation spectroscopy.
Kahya N, Scherfeld D, Bacia K, Schwille P. Kahya N, et al. J Struct Biol. 2004 Jul;147(1):77-89. doi: 10.1016/j.jsb.2003.09.021. J Struct Biol. 2004. PMID: 15109608 Review.
Cited by
- Quantifying disorder through conditional entropy: an application to fluid mixing.
Brandani GB, Schor M, Macphee CE, Grubmüller H, Zachariae U, Marenduzzo D. Brandani GB, et al. PLoS One. 2013 Jun 10;8(6):e65617. doi: 10.1371/journal.pone.0065617. Print 2013. PLoS One. 2013. PMID: 23762401 Free PMC article. - Crowding-Induced Mixing Behavior of Lipid Bilayers: Examination of Mixing Energy, Phase, Packing Geometry, and Reversibility.
Zeno WF, Rystov A, Sasaki DY, Risbud SH, Longo ML. Zeno WF, et al. Langmuir. 2016 May 10;32(18):4688-97. doi: 10.1021/acs.langmuir.6b00831. Epub 2016 Apr 26. Langmuir. 2016. PMID: 27096947 Free PMC article. - FCS diffusion laws in two-phase lipid membranes: determination of domain mean size by experiments and Monte Carlo simulations.
Favard C, Wenger J, Lenne PF, Rigneault H. Favard C, et al. Biophys J. 2011 Mar 2;100(5):1242-51. doi: 10.1016/j.bpj.2010.12.3738. Biophys J. 2011. PMID: 21354397 Free PMC article. - Phase separation and fluctuations in mixtures of a saturated and an unsaturated phospholipid.
Svetlovics JA, Wheaten SA, Almeida PF. Svetlovics JA, et al. Biophys J. 2012 Jun 6;102(11):2526-35. doi: 10.1016/j.bpj.2012.04.017. Epub 2012 Jun 5. Biophys J. 2012. PMID: 22713568 Free PMC article. - Lipid bilayer membrane-triggered presynaptic vesicle assembly.
Gopalakrishnan G, Thostrup P, Rouiller I, Lucido AL, Belkaïd W, Colman DR, Lennox RB. Gopalakrishnan G, et al. ACS Chem Neurosci. 2010 Feb 17;1(2):86-94. doi: 10.1021/cn900011n. Epub 2009 Oct 15. ACS Chem Neurosci. 2010. PMID: 22778819 Free PMC article.
References
- Almeida, P. F. F., and W. L. C. Vaz. 1995. Lateral diffusion in membranes. In Structure and Dynamics of Membranes: From Cells to Vesicles. R. Lipowski and E. Sackmann, editors. Elsevier, Amsterdam, The Netherlands. 305–357.
- Almeida, P. F. F., W. L. C. Vaz, and T. E. Thompson. 1992a. Lateral diffusion and percolation in two-phase, two-component lipid bilayers. Topology of the solid-phase domains in-plane and across the lipid bilayer. Biochemistry. 31:7198–7210. - PubMed
- Almeida, P. F. F., W. L. C. Vaz, and T. E. Thompson. 1992b. Lateral diffusion in the liquid phases of dimyristoylphosphatidylcholine/cholesterol lipid bilayers: a free volume analysis. Biochemistry. 31:6739–6747. - PubMed
- Angelova, M. I., S. Soleau, P. Meleard, J. F. Faucon, and P. Bothorel. 1992. Preparation of giant vesicles by external AC electric fields. Progr. Colloid Polym. Sci. 89:127–131.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources