DJ-1 is a redox-dependent molecular chaperone that inhibits alpha-synuclein aggregate formation - PubMed (original) (raw)
DJ-1 is a redox-dependent molecular chaperone that inhibits alpha-synuclein aggregate formation
Shoshana Shendelman et al. PLoS Biol. 2004 Nov.
Abstract
Parkinson's disease (PD) pathology is characterized by the degeneration of midbrain dopamine neurons (DNs) ultimately leading to a progressive movement disorder in patients. The etiology of DN loss in sporadic PD is unknown, although it is hypothesized that aberrant protein aggregation and cellular oxidative stress may promote DN degeneration. Homozygous mutations in DJ-1 were recently described in two families with autosomal recessive inherited PD (Bonifati et al. 2003). In a companion article (Martinat et al. 2004), we show that mutations in DJ-1 alter the cellular response to oxidative stress and proteasomal inhibition. Here we show that DJ-1 functions as a redox-sensitive molecular chaperone that is activated in an oxidative cytoplasmic environment. We further demonstrate that DJ-1 chaperone activity in vivo extends to alpha-synuclein, a protein implicated in PD pathogenesis.
Conflict of interest statement
The authors have declared that no conflicts of interest exist.
Figures
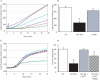
Figure 1. DJ-1 Is a Redox-Dependent Molecular Chaperone
(A) Aggregation of CS was monitored at 43 °C after addition of either 0.8 μM CS alone (black), or along with 8.0 μM RNase A (purple), 0.5 μM DJ-1 (aqua), 2.0 μM DJ-1 (blue), 4.0 μM DJ-1 (red), or 2.0 μM Hsp27 (green). (B) Aggregation of 0.8 μM CS after 30 min at 4 °C (unfilled bar) is inhibited by 4.0 μM WT DJ-1 (black bar) but not 4.0 μM L166P mutant DJ-1 (gray bar). Data are shown as the mean ± SEM and were analyzed by ANOVA with Fisher's post-hoc test. * p < 0.05. (C) Aggregation of insulin (26 μM) B chains induced by 20 mM DTT at 25 °C. Insulin alone (black) or in the presence of 4.0 μM RNase A (purple), 0.5 μM DJ-1 (aqua), 2.0 μM DJ-1 (blue), 4.0 μM DJ-1 (red), or 2.0 μM Hsp27 (green). (D) CS thermal aggregation (unfilled bar) is suppressed by 4 μM DJ-1 (black bar), but chaperone activity is abrogated upon incubation of DJ-1 with 0.5 mM DTT for 10 min at 4 °C (gray bar). Further treatment of DTT-reduced DJ-1 with 10 mM H2O2 for 10 min at 4 °C leads to reactivation of CS suppression (hatched bar). Data are shown as the mean ± SEM and were analyzed by ANOVA with Fisher's post-hoc test. * p < 0.05.
Figure 2. DJ-1 Inhibits Formation of αSyn Protofibrils and Fibrils In Vitro
(A) Purified αSyn (200 μM) was incubated for 2 h at 55 °C in the presence of WT DJ-1, L166P mutant DJ-1, GST, or Hsp27 (all at 100 μM). WT DJ-1 inhibits accumulation of αSyn protofibrils in vitro, while L166P mutant DJ-1, GST, and Hsp27 do not. (B) Suppression of αSyn protofibril formation by WT DJ-1 (in triplicate) was quantified as compared to GST (as a negative control) and mutant L166P DJ-1. Data are shown as the mean ± SEM and were analyzed by ANOVA with Fisher's post-hoc test. * p < 0.05. (C) Purified αSyn (200 μM) was incubated for 1 wk at 37 °C in the presence of WT DJ-1, L166P mutant DJ-1, or GST (all at 100 μM). WT DJ-1 inhibits formation of mature Congo red–positive αSyn fibrils. Data are shown as the mean ± SEM and were analyzed by ANOVA with Fisher's post-hoc test. * p < 0.05.
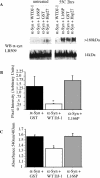
Figure 3. Overexpression of WT DJ-1 Inhibits Aggregation of αSyn In Vivo
(A) CAD murine neuroblastoma cells were transfected with Flag-αSyn along with WT DJ-1, L166P clinical mutant, or vector alone, and were differentiated in vitro via serum withdrawal. Cells were subsequently treated with 2 mM FeCl2 (Fe), 5 μM lactacystin (LC), or media alone (0). Triton X-100-soluble (Tx-100 sol) and Triton X-100-insoluble (Tx-100 insol) fractions were analyzed by Western blotting. Upon FeCl2 treatment, αSyn accumulates in the Triton X-100-insoluble fraction, and accumulation of insoluble αSyn is inhibited by overexpression of WT DJ-1 (left) but not the L166P clinical mutant (right). (B) Triton X-100-insoluble αSyn as quantified by NIH Image J of a Western blot (from [A]). (C) Heterozygous (+/–) and DJ-1 deficient (–/–) ES cells were differentiated using the embryoid body protocol. Cells were transfected with Flag-αSyn (F-αSyn), and, after 48 h, treated with 2 mM FeCl2 or with media alone for 18 h. Cell lysates were analyzed by Western blotting for αSyn or β-actin. In the Triton X-100-soluble fraction (Tx-100 sol), DJ-1 accumulated to a similar extent in the knockout and control cells. In contrast, αSyn accumulation in the insoluble pool (Tx-100 insol) was detectable only in the knockout cells, and this was further promoted by FeCl2 treatment. (D) CAD cells transfected with Flag-αSyn (F-αSyn) along with WT DJ-1 (or vector alone) were treated with 2 mM FeCl2 or media alone for 18 h. Triton X-100-soluble cell lysates were immunoprecipitated with a mouse monoclonal antibody for the Flag epitope and Western blotted for DJ-1. FeCl2 treatment induces association of Flag-αSyn with WT DJ-1. Lysates represent 20% input of the immunoprecipitation (IP α-Flag). The Triton X-100 soluble pool of DJ-1 is reduced by αSyn overexpression (but not vector control), particularly in the context of FeCl2 treatment (bottom). (E) DJ-1 colocalizes with αSyn in the Triton X-100-insoluble fraction upon FeCl2 treatment. The Western blot from (A) was stripped and reprobed for DJ-1.
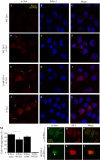
Figure 4. DJ-1 Inhibits Formation of αSyn Intracytoplasmic Inclusions
(A–L) CAD murine neuroblastoma cells were transfected with WT DJ-1 (A–F), L166P DJ-1 (G–I) or vector control (J–L), along with Flag-αSyn (D–L) or vector control (A–C) and differentiated in vitro by serum withdrawal for 72 h. Cells were fixed and stained with a mouse monoclonal antibody for αSyn and ToPro3, a nuclear dye, and images were obtained by confocal microscopy. Transfection of Flag-αSyn induced formation of intracytoplasmic inclusions (arrows). Scale bar, 20 μm. (M) Quantification of cells with inclusions was performed on ten random images from each of three wells per condition. Images were quantified by an observer blinded to the experiment. A significantly lower percentage of cells harbor inclusions in the context of WT DJ-1 overexpression. Aggregation is expressed as the percentage of cells containing αSyn aggregates per frame. Total cell number per frame, as determined by ToPro3 staining, did not differ significantly (Figure S3). Data are shown as the mean ± SEM, and were analyzed by ANOVA with Fisher's post-hoc test. * p < 0.05. (N–S) Cells were fixed and stained with a monoclonal antibody for αSyn and a polyclonal antibody that recognizes both transfected human DJ-1 and endogenous murine DJ-1. DJ-1 does not appear to colocalize with the αSyn aggregates. Scale bar, 20 μm.
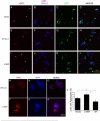
Figure 5. DJ-1 Inhibits Formation of NFL Intracytoplasmic Inclusions
(A–L) CAD cells were transfected with an aggregation-prone mutant NFL (Q333P) plasmid, as well as WT human DJ-1 plasmid (that also harbors GFP; E–H), L166P mutant DJ-1 (that also harbors GFP; I–L), or control GFP vector (A–D). After 72 h in culture, cells were fixed and stained with a mouse monoclonal antibody for NFL and ToPro3, a nuclear dye. Scale bar, 100 μm. (M–R) CAD cell transfectants, as above, were fixed and stained with a polyclonal antibody for NFL (Perez-Olle et al. 2002) along with a mouse monoclonal antibody specific for the transfected human DJ-1. Scale bar, 20 μm. (S) Quantification of CAD cell NFL aggregates was performed using confocal microscopy. Images from tenrandomly selected fields in each of three wells were quantified for the presence of aggregates for each condition and presented as a percentage of total cells per field. Total cell number was determined by ToPro3 nuclear staining and did not differ significantly (Figure S3). Data are shown as the mean ± SEM and were analyzed by ANOVA with Fisher's post-hoc test. * p < 0.05.
Figure 6. DJ-1 In Vitro Chaperone Activity and In Vivo Oxidative Stress Protection Activity Require Cysteine 53 but Not Cysteine 106
(A) DJ-1 cysteine-to-alanine mutants C106A, C53A, and a triple mutant that harbors mutations at all three cysteines in DJ-1 (C106A/C53A/C46A), as well as L166P, were tested for in vitro chaperone activity by CS aggregation suppression assay. (B) Self-association of DJ-1 cysteine mutants. Murine neuroblastoma CAD cells were transiently cotransfected with Flag-tagged human DJ-1 vectors (either WT or mutant) along with WT YFP-tagged human DJ-1. Lysates were immunoprecipitated with anti-Flag antibodies and probed by Western blotting with an antibody specific for human DJ-1. WT Flag-DJ-1, C106A DJ-1, C53A DJ-1, and C106A/C53A/C46A DJ-1 effectively coprecipitated WT GFP-DJ-1, whereas the L166P mutant Flag-DJ-1 failed to do so. Lysates represent 20% of the input for the immunoprecipitate; Flag-DJ-1 migrates at 22 kDa, and YFP-DJ-1 migrates at 50 kDa. (C) DJ-1-deficient ES cells were transiently transfected with vector alone, WT DJ-1, or DJ-1 cysteine mutants, and exposed to 10 μM H2O2 for 15 h followed by MTT assay. The viability of the cells in the absence of drug treatment was not altered by the expression of WT or mutant DJ-1). Data are shown as the mean ± SEM and were analyzed by ANOVA with Fisher's post-hoc test. * p < 0.05 (D) Expression levels of WT and mutant forms of DJ-1 were comparable as determined by Western blotting for human DJ-1 and β-actin.
Similar articles
- The oxidation state of DJ-1 regulates its chaperone activity toward alpha-synuclein.
Zhou W, Zhu M, Wilson MA, Petsko GA, Fink AL. Zhou W, et al. J Mol Biol. 2006 Mar 3;356(4):1036-48. doi: 10.1016/j.jmb.2005.12.030. Epub 2005 Dec 27. J Mol Biol. 2006. PMID: 16403519 - DJ-1 up-regulates glutathione synthesis during oxidative stress and inhibits A53T alpha-synuclein toxicity.
Zhou W, Freed CR. Zhou W, et al. J Biol Chem. 2005 Dec 30;280(52):43150-8. doi: 10.1074/jbc.M507124200. Epub 2005 Oct 14. J Biol Chem. 2005. PMID: 16227205 - Sensitivity to oxidative stress in DJ-1-deficient dopamine neurons: an ES- derived cell model of primary Parkinsonism.
Martinat C, Shendelman S, Jonason A, Leete T, Beal MF, Yang L, Floss T, Abeliovich A. Martinat C, et al. PLoS Biol. 2004 Nov;2(11):e327. doi: 10.1371/journal.pbio.0020327. Epub 2004 Oct 5. PLoS Biol. 2004. PMID: 15502868 Free PMC article. - Impaired mitochondrial dynamics and function in the pathogenesis of Parkinson's disease.
Büeler H. Büeler H. Exp Neurol. 2009 Aug;218(2):235-46. doi: 10.1016/j.expneurol.2009.03.006. Epub 2009 Mar 18. Exp Neurol. 2009. PMID: 19303005 Review. - New aspects of genetic contributions to Parkinson's disease.
Hofer A, Gasser T. Hofer A, et al. J Mol Neurosci. 2004;24(3):417-24. doi: 10.1385/JMN:24:3:417. J Mol Neurosci. 2004. PMID: 15655263 Review.
Cited by
- Genotype-specific differences between mouse CNS stem cell lines expressing frontotemporal dementia mutant or wild type human tau.
Orr ME, Pitstick R, Canine B, Ashe KH, Carlson GA. Orr ME, et al. PLoS One. 2012;7(6):e39328. doi: 10.1371/journal.pone.0039328. Epub 2012 Jun 18. PLoS One. 2012. PMID: 22723997 Free PMC article. - Retigeric acid B-induced mitophagy by oxidative stress attenuates cell death against prostate cancer cells in vitro.
Liu YQ, Ji Y, Li XZ, Tian KL, Young CY, Lou HX, Yuan HQ. Liu YQ, et al. Acta Pharmacol Sin. 2013 Sep;34(9):1183-91. doi: 10.1038/aps.2013.68. Epub 2013 Jul 29. Acta Pharmacol Sin. 2013. PMID: 23892275 Free PMC article. - Genetic mouse models of parkinsonism: strengths and limitations.
Fleming SM, Fernagut PO, Chesselet MF. Fleming SM, et al. NeuroRx. 2005 Jul;2(3):495-503. doi: 10.1602/neurorx.2.3.495. NeuroRx. 2005. PMID: 16389313 Free PMC article. Review. - Rewiring of the Human Mitochondrial Interactome during Neuronal Reprogramming Reveals Regulators of the Respirasome and Neurogenesis.
Moutaoufik MT, Malty R, Amin S, Zhang Q, Phanse S, Gagarinova A, Zilocchi M, Hoell L, Minic Z, Gagarinova M, Aoki H, Stockwell J, Jessulat M, Goebels F, Broderick K, Scott NE, Vlasblom J, Musso G, Prasad B, Lamantea E, Garavaglia B, Rajput A, Murayama K, Okazaki Y, Foster LJ, Bader GD, Cayabyab FS, Babu M. Moutaoufik MT, et al. iScience. 2019 Sep 27;19:1114-1132. doi: 10.1016/j.isci.2019.08.057. Epub 2019 Sep 4. iScience. 2019. PMID: 31536960 Free PMC article. - Protein aggregation in a mutant deficient in yajL, the bacterial homolog of the Parkinsonism-associated protein DJ-1.
Kthiri F, Le HT, Gautier V, Caldas T, Malki A, Landoulsi A, Bohn C, Bouloc P, Richarme G. Kthiri F, et al. J Biol Chem. 2010 Apr 2;285(14):10328-36. doi: 10.1074/jbc.M109.077529. Epub 2010 Feb 2. J Biol Chem. 2010. PMID: 20124404 Free PMC article.
References
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, et al. Mice lacking α-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. - PubMed
- Beal MF. Oxidatively modified proteins in aging and disease. Free Radic Biol Med. 2002;32:797–803. - PubMed
- Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. - PubMed
- Chelikani P, Donald LJ, Duckworth HW, Loewen PC. Hydroperoxidase II of Escherichia coli exhibits enhanced resistance to proteolytic cleavage compared to other catalases. Biochemistry. 2003;42:5729–5735. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous