Evaluation of the 17-kDa prenyl-binding protein as a regulatory protein for phototransduction in retinal photoreceptors - PubMed (original) (raw)
Evaluation of the 17-kDa prenyl-binding protein as a regulatory protein for phototransduction in retinal photoreceptors
Angela W Norton et al. J Biol Chem. 2005.
Abstract
The mammalian rod photoreceptor phosphodiesterase (PDE6) holoenzyme is isolated in both a membrane-associated and a soluble form. Membrane binding is a consequence of prenylation of PDE6 catalytic subunits, whereas soluble PDE6 is purified with a 17-kDa prenyl-binding protein (PDEdelta) tightly bound. This protein, here termed PrBP/delta, has been hypothesized to reduce activation of PDE6 by transducin, thereby desensitizing the photoresponse. To test the potential role of PrBP/delta in regulating phototransduction, we examined the abundance, localization, and potential binding partners of PrBP/delta in retina and in purified rod outer segment (ROS) suspensions whose physiological and biochemical properties are well characterized. The amphibian homologue of PrBP/delta was cloned and sequenced and found to have 82% amino acid sequence identity with mammalian PrBP/delta. In contrast to bovine ROS, all of the PDE6 in purified frog ROS is membrane-associated. However, addition of recombinant frog PrBP/delta can solubilize PDE6 and prevent its activation by transducin. PrBP/delta also binds other prenylated photoreceptor proteins in vitro, including opsin kinase (GRK1/GRK7) and rab8. Quantitative immunoblot analysis of the PrBP/delta content of purified ROS reveals insufficient amounts of PrBP/delta (<0.1 PrBP/delta per PDE6) to serve as a subunit of PDE6 in either mammalian or amphibian photoreceptors. The immunolocalization of PrBP/delta in frog and bovine retina shows greatest PrBP/delta immunolabeling outside the photoreceptor cell layer. Within photoreceptors, only the inner segments of frog double cones are strongly labeled, whereas bovine photoreceptors reveal more PrBP/delta labeling near the junction of the inner and outer segments (connecting cilium) of photoreceptors. Together, these results rule out PrBP/delta as a PDE6 subunit and implicate PrBP/delta in the transport and membrane targeting of prenylated proteins (including PDE6) from their site of synthesis in the inner segment to their final destination in the outer segment of rods and cones.
Figures
Fig. 1. The frog homolog of mammalian PrBP/δ is highly conserved
The amino acid sequence of R. pipiens PrBP/δ was aligned and compared with eight other vertebrate sequences: bovine (shown), human, dog, rat, mouse, chick, fugu (partial sequence from genomic data base), and zebrafish (from genomic data base). Residues in red are identical in at least eight out of nine vertebrate species. Multiple sequence alignment was also performed, including six invertebrate, full-length PrBP/δ orthologous sequences: C. elegans, C. briggsae, C. intestinalis, D. melanogaster, A. gambiae, and Halocynthia roretzi. Residues in boldface red are identical in at least 14 out of 15 species. Secondary structure (above sequence) is based on the PrBP/δ crystal structure, and sites that are structurally equivalent to the prenyl interacting sites in the crystal structure of RhoGDI are boxed (9).
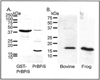
Fig. 2. Recombinant expression of frog PrBP/δ and antibody specificity of the PrBP/δ antibody (FL)
A, bacterially expressed frog GST-PrBP/δ fusion protein was purified by glutathione agarose affinity chromatography and then treated with thrombin to cleave the fusion partner from PrBP/δ. Samples were analyzed by SDS-PAGE and Coomassie staining. Lane 1: 20 µg of frog GST-PrBP/δ. Lane 2: 20 µg of PrBP/δ. B, the specificity of the PrBP/δ antibody (FL) was tested by immunoblot analysis of bovine and frog retinal extracts (11 µg of protein) run on SDS-PAGE, transferred to polyvinylidene difluoride membranes, and the membranes probed with 1:1000 dilution of the FL antibody and 1:3000 dilution of secondary antibody.
Fig. 3. Recombinant frog PrBP/δ solubilizes PDE6 from ROS membranes and forms a stable complex in vitro
A, purified frog ROS homogenates (30 n
m
PDE) were incubated with increasing amounts of recombinant frog PrBP/δ for ≥17 h at 4 °C. Each sample was centrifuged to separate membrane-associated (●) from soluble (○) PDE6. The PDE6 concentration was determined by quantifying cGMP binding (see “Experimental Procedures”) and normalized to the PDE6 concentration in the absence of added PrBP/δ (<5% soluble). Data are mean ± S.E. for six separate experiments. B, frog ROS homogenates were incubated overnight at 4 °C with an 8-fold molar excess of frog PrBP/δ relative to PDE6. Solubilized PDE6 was separated from ROS membranes by centrifugation. ROS1-Sulfolink was then incubated with the supernatant fraction for 2 h at 22 °C. Precipitated proteins were recovered by brief centrifugation of the antibody beads. The pellet and supernatant fractions were mixed with SDS-PAGE gel sample buffer, electrophoresed, and transferred to membranes. The immunoblot was probed with a mixture of PrBP/δ (FL) and PDE6 (NC) antibodies. HC and LC refer to IgG heavy and light chains, which cross-reacted with the secondary reagent.
Fig. 4. PrBP/δ blocks transducin activation of PDE6 in vitro without releasing Tα*-GTPγS from ROS membranes
A, nucleotide-depleted, light-exposed frog ROSs (40 n
m
PDE6) were incubated overnight at 4 °C with the indicated amount of PrBP/δ. To one portion (●), 13 µ
m
GTPγS was added to determine the maximum extent to which PDE6 could be activated by transducin. Another portion (○) was centrifuged to determine the extent to which non-activated PDE6 was solubilized (as in Fig. 3). B, immunoblot analysis of ROS homogenates treated with the indicated amounts of PrBP/δ prior to centrifugation. Supernatants (S) and membrane pellets (M) were analyzed on immunoblots using a transducin (_T_α) antibody.
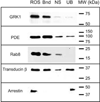
Fig. 5. PrBP/δ binds to several prenylated photoreceptor proteins in bovine ROS homogenates
Detergent-solubilized bovine ROS (3.5 pmol of PDE6) were incubated with PrBP/δ-Sepharose (2 mg of PrBP/δ per milliliter of resin), and proteins bound to the resin (Bnd) were separated from unbound proteins (UB) by centrifugation. As a control for nonspecific binding to the resin (NS), identical samples were incubated with a large excess of PrBP/δ. Equivalent amounts of the starting sample (ROS) were also loaded. After SDS-PAGE and transfer to polyvinylidene difluoride, blots were probed with primary antibodies to GRK1, PDE6, Rab8, transducin β-subunit, and arrestin.
Fig. 6. Quantitating PrBP/δ content in retinal extracts and in ROS
A, detergent-treated frog retinal extracts (1.0 pmol of PDE6) and Percoll-purified frog ROS (1.0 pmol of PDE6) were examined for their PrBP/δ content by reference to the indicated amounts of purified, recombinant frog PrBP/δ. B, detergent-treated bovine retinal extracts (0.25 pmol of PDE6) and sucrose-purified ROS (0.5 pmol of PDE6) were analyzed as in panel A.
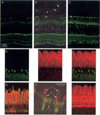
Fig. 7. Immunohistochemical localization of PrBP/δ in frog retina
Frog retina was isolated, fixed, and sectioned as described under “Experimental Procedures” and incubated with primary antibodies to the indicated proteins: A, PrBP/δ antibody (FL, green). B, same as A with phase contrast superimposed and retinal layers identified: OS, outer segment; RIS, rod inner segment; ONL, outer nuclear layer, OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. Stain appears greatest in certain inner segments and stratum 1 of the IPL. Arrowheads indicate cone outer segments, while arrows indicate rod outer segment. C, FL antibody signal amplified with biotin-streptavidin detection reagents permits labeling of all rod inner segments (arrowhead). D–F, double labeling with FL (green) and ROS1 (a PDE6 catalytic subunit antibody, red) stainings do not co-localize. G, superimposition of PDE6 (ROS1, red) and Pγ-subunit (CT-9710, green) show co-localization in the outer segments. H, superimposition of phase contrast image with FL (green) and red/green cone opsin (COS-1, red). Arrows point to two double cones: pc, principal cone; ac, accessory cone. Arrowhead points to rod inner segment (RIS). I, double staining of FL (green) and rod arrestin (SCT-128, red).
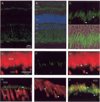
Fig. 8. Immunohistochemical localization of PrBP/δ in bovine retina
Light-adapted bovine retinas were fixed and sectioned as described under “Experimental Procedures,” and then sections were incubated with the following primary antibodies. Arrowheads in some panels indicate the connecting cilium (cc) region. A, PrBP/δ (FL, green) alone. B, same as A with superimposition of phase contrast image and 4′,6-diamidino-2-phenylindole (blue) staining, with layers identified as in Fig. 7. C, FL staining of connecting cilium region of rods (upper arrowhead) and cones (lower arrowhead). D, superimposition of C with phase contrast image. E–G, double labeling of PDE6 antibody (ROS1, red) and FL (green); PrBP/δ-staining is greatest at the base of the outer segment labeled by ROS1. H, cone arrestin (7G6, red) stains cones (COS and CIS) but not rods, whereas FL staining (green) in cones is at the junction of COS and CIS. I, GRK1/7 (G8, red) stains rod and cone outer segments, whereas FL staining (green) is observed at the base of outer segments. J, RP1 (anti-RP1, red) stains the connecting cilium region and overlaps with tyramide-amplified FL staining (green).
Fig. 9. Immunoelectron microscopy localizes PrBP/δ to the ciliary axonemes of bovine rods and cones
A, semithin sections from bovine retina were stained for PrBP/δ (see “Experimental Procedures”). Immunoreactivity concentrated in the connecting cilium region (arrowheads) that joins cone inner segments (cis) and outer segments (cos) or rod inner segments (ris) and outer segments. B and C, ultrathin sections stained for PrBP/δ. Labeling is present in both rod (B) and cone (C) axonemal microtubules. Stain is greatest above the basal body (bb) and connecting cilium (cc), where it extends up along the axonemes (brackets).
Similar articles
- Assay and functional properties of PrBP(PDEdelta), a prenyl-binding protein interacting with multiple partners.
Zhang H, Hosier S, Terew JM, Zhang K, Cote RH, Baehr W. Zhang H, et al. Methods Enzymol. 2005;403:42-56. doi: 10.1016/S0076-6879(05)03005-3. Methods Enzymol. 2005. PMID: 16473576 - Deletion of PrBP/delta impedes transport of GRK1 and PDE6 catalytic subunits to photoreceptor outer segments.
Zhang H, Li S, Doan T, Rieke F, Detwiler PB, Frederick JM, Baehr W. Zhang H, et al. Proc Natl Acad Sci U S A. 2007 May 22;104(21):8857-62. doi: 10.1073/pnas.0701681104. Epub 2007 May 11. Proc Natl Acad Sci U S A. 2007. PMID: 17496142 Free PMC article. - Active transducin alpha subunit carries PDE6 to detergent-resistant membranes in rod photoreceptor outer segments.
Liu H, Seno K, Hayashi F. Liu H, et al. Biochem Biophys Res Commun. 2003 Mar 28;303(1):19-23. doi: 10.1016/s0006-291x(03)00284-5. Biochem Biophys Res Commun. 2003. PMID: 12646160 - A model for transport of membrane-associated phototransduction polypeptides in rod and cone photoreceptor inner segments.
Karan S, Zhang H, Li S, Frederick JM, Baehr W. Karan S, et al. Vision Res. 2008 Feb;48(3):442-52. doi: 10.1016/j.visres.2007.08.020. Epub 2007 Oct 18. Vision Res. 2008. PMID: 17949773 Free PMC article. Review. - The prenyl-binding protein PrBP/δ: a chaperone participating in intracellular trafficking.
Zhang H, Constantine R, Frederick JM, Baehr W. Zhang H, et al. Vision Res. 2012 Dec 15;75:19-25. doi: 10.1016/j.visres.2012.08.013. Epub 2012 Aug 29. Vision Res. 2012. PMID: 22960045 Free PMC article. Review.
Cited by
- Identification of a novel prenyl and palmitoyl modification at the CaaX motif of Cdc42 that regulates RhoGDI binding.
Nishimura A, Linder ME. Nishimura A, et al. Mol Cell Biol. 2013 Apr;33(7):1417-29. doi: 10.1128/MCB.01398-12. Epub 2013 Jan 28. Mol Cell Biol. 2013. PMID: 23358418 Free PMC article. - Structural characterization of the rod cGMP phosphodiesterase 6.
Goc A, Chami M, Lodowski DT, Bosshart P, Moiseenkova-Bell V, Baehr W, Engel A, Palczewski K. Goc A, et al. J Mol Biol. 2010 Aug 20;401(3):363-73. doi: 10.1016/j.jmb.2010.06.044. Epub 2010 Jul 1. J Mol Biol. 2010. PMID: 20600113 Free PMC article. - Light-dependent compartmentalization of transducin in rod photoreceptors.
Artemyev NO. Artemyev NO. Mol Neurobiol. 2008 Feb;37(1):44-51. doi: 10.1007/s12035-008-8015-2. Epub 2008 Apr 19. Mol Neurobiol. 2008. PMID: 18425604 Review. - Phosphodiesterases maintain signaling fidelity via compartmentalization of cyclic nucleotides.
Lomas O, Zaccolo M. Lomas O, et al. Physiology (Bethesda). 2014 Mar;29(2):141-9. doi: 10.1152/physiol.00040.2013. Physiology (Bethesda). 2014. PMID: 24583770 Free PMC article. Review. - Mechanisms of mutant PDE6 proteins underlying retinal diseases.
Gopalakrishna KN, Boyd K, Artemyev NO. Gopalakrishna KN, et al. Cell Signal. 2017 Sep;37:74-80. doi: 10.1016/j.cellsig.2017.06.002. Epub 2017 Jun 2. Cell Signal. 2017. PMID: 28583373 Free PMC article.
References
- Arshavsky VY, Lamb TD, Pugh EN., Jr Annu. Rev. Physiol. 2002;64:153–187. - PubMed
- Stavenga DG, DeGrip WJ, Pugh EN., Jr . Molecular Mechanisms in Visual Transduction. Amsterdam: Elsevier Science; 2002.
- Cote RH. In: Handbook of Cell Signaling. Bradshaw RA, Dennis EA, editors. San Diego, CA: Academic Press; 2003. pp. 453–458.
- Gillespie PG, Beavo JA. J. Biol. Chem. 1988;263:8133–8141. - PubMed
- Gillespie PG, Prusti RK, Apel ED, Beavo JA. J. Biol. Chem. 1989;264:12187–12193. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- EY05798/EY/NEI NIH HHS/United States
- EY08123/EY/NEI NIH HHS/United States
- R01 EY008123/EY/NEI NIH HHS/United States
- R01 EY011105/EY/NEI NIH HHS/United States
- R01 EY005798/EY/NEI NIH HHS/United States
- EY11105/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous