The gut microbiota as an environmental factor that regulates fat storage - PubMed (original) (raw)
The gut microbiota as an environmental factor that regulates fat storage
Fredrik Bäckhed et al. Proc Natl Acad Sci U S A. 2004.
Abstract
New therapeutic targets for noncognitive reductions in energy intake, absorption, or storage are crucial given the worldwide epidemic of obesity. The gut microbial community (microbiota) is essential for processing dietary polysaccharides. We found that conventionalization of adult germ-free (GF) C57BL/6 mice with a normal microbiota harvested from the distal intestine (cecum) of conventionally raised animals produces a 60% increase in body fat content and insulin resistance within 14 days despite reduced food intake. Studies of GF and conventionalized mice revealed that the microbiota promotes absorption of monosaccharides from the gut lumen, with resulting induction of de novo hepatic lipogenesis. Fasting-induced adipocyte factor (Fiaf), a member of the angiopoietin-like family of proteins, is selectively suppressed in the intestinal epithelium of normal mice by conventionalization. Analysis of GF and conventionalized, normal and Fiaf knockout mice established that Fiaf is a circulating lipoprotein lipase inhibitor and that its suppression is essential for the microbiota-induced deposition of triglycerides in adipocytes. Studies of Rag1-/- animals indicate that these host responses do not require mature lymphocytes. Our findings suggest that the gut microbiota is an important environmental factor that affects energy harvest from the diet and energy storage in the host. Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. AY 667702--AY 668946).
Figures
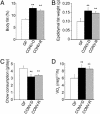
Fig. 1.
Phenotyping WT gnotobiotic mice. Three groups of 8- to 10-week-old adult male B6 mice [those raised in a GF state, those allowed to acquire a microbiota from birth to adulthood (CONV-R), and those raised GF until adulthood and then colonized for 2 weeks with an unfractionated cecal microbiota harvested from CONV-R donors (CONV-D)] were analyzed for total body fat content by dual energy x-ray absorptiometry (n = 21-25 per group) (A), epididymal fat weight (n = 10-20 per group) (B), chow consumption (average daily value over the 3 d before termination of the experiment; n = 10 per group) (C), and oxygen consumption (defined by open circuit calorimetry just before the animals were killed; n = 10 per group) (D). Mean values ± SEM are plotted. **, P < 0.01 compared with GF.
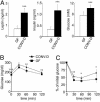
Fig. 2.
A 14-d conventionalization of WT GF B6 mice increases circulating leptin levels and decreases sensitivity to insulin. (A) Sera were obtained after a 4-h fast and analyzed for leptin, insulin, and glucose (n = 8 animals per group). Numbers represent mean values ± SEM. Glucose tolerance (B) and insulin tolerance (C) tests were performed after a 4-h fast (n = 8 mice per group). Mean values ± SEM are plotted. ***, P < 0.001; **, P < 0.01; and *, P < 0.05 compared with GF.
Fig. 3.
Conventionalization induces hepatic lipogenesis and nuclear import of the basic helix-loop-helix transcription factor ChREBP. (A) Oil-red O stains of paraformaldehyde-fixed liver sections prepared from 8-week-old WT male GF and CONV-D B6 mice. (B) Liver triglyceride (TG) levels (n = 5 per group; mean values ± SEM; ***, P < 0.001 compared to GF). (C) qRT-PCR assays of liver RNAs from GF and CONV-D mice [n = 15 per group; mean values ± SEM are expressed relative to levels in GF animals (GF set at 100%); *, P < 0.05; ***, P < 0.001 compared with GF]. (D) Immunohistochemical study of paraformaldehyde-fixed sections of livers from GF or CONV-D mice. Sections were stained with rabbit polyclonal antibodies to mouse ChREBP (green). Nuclei are labeled dark blue with 4′,6-diamidino-2-phenylindole. (Bars: 25 μm.)
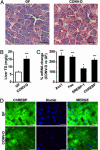
Fig. 4.
Conventionalization promotes adipocyte hypertrophy by suppressing Fiaf expression in the intestine. (A Upper) Epididymal fat pads from 8-week-old WT male GF, CONV-D, and CONV-R B6 mice. (A Lower) The corresponding hematoxylin- and eosin-stained sections are shown. (B) qRT-PCR assays of epididymal fat pad RNAs harvested from WT mice reveal that conventionalization does not produce significant changes in expression of mediators or biomarkers of lipogenesis and adipogenesis (mean values ± SEM are plotted; P > 0.05; n = 15 per group). (C) LPL activity is increased upon conventionalization in both epididymal fat pads and heart of WT mice (**, P < 0.01 and ***, P < 0.001 compared to GF; n = 5 per group). (D) qRT-PCR assays of Fiaf expression in WT animals [***, P < 0.001 compared to GF (set at 100%); n = 13]. (E) Generation of Fiaf knockout mice. Structures are shown for the WT Fiaf locus, the targeting vector, and the mutated locus with exons 1-3 replaced by a βgeopA cassette. The desired disruption was verified by Southern blot analysis. Northern blots of adipocyte RNA establish the absence of detectable Fiaf mRNA in _Fiaf_-/- animals. (F) The absence of Fiaf markedly attenuates the increase in total body fat content after a 14-d conventionalization (**, P < 0.01 compared with WT; n = 7-8 per group).
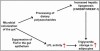
Fig. 5.
Schematic view of how the gut microbiota effects host fat storage. The microbiota acts through Fiaf to coordinate increased hepatic lipogenesis with increased LPL activity in adipocytes, thereby promoting storage of calories harvested from the diet into fat. See text for further details.
Similar articles
- Absence of intestinal microbiota does not protect mice from diet-induced obesity.
Fleissner CK, Huebel N, Abd El-Bary MM, Loh G, Klaus S, Blaut M. Fleissner CK, et al. Br J Nutr. 2010 Sep;104(6):919-29. doi: 10.1017/S0007114510001303. Epub 2010 May 5. Br J Nutr. 2010. PMID: 20441670 - Preserved adiposity in the Fischer 344 rat devoid of gut microbiota.
Swartz TD, Sakar Y, Duca FA, Covasa M. Swartz TD, et al. FASEB J. 2013 Apr;27(4):1701-10. doi: 10.1096/fj.12-221689. Epub 2013 Jan 24. FASEB J. 2013. PMID: 23349551 - Mechanisms underlying the resistance to diet-induced obesity in germ-free mice.
Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI. Bäckhed F, et al. Proc Natl Acad Sci U S A. 2007 Jan 16;104(3):979-84. doi: 10.1073/pnas.0605374104. Epub 2007 Jan 8. Proc Natl Acad Sci U S A. 2007. PMID: 17210919 Free PMC article. - Gut microbiota: a factor in energy regulation.
Wolf G. Wolf G. Nutr Rev. 2006 Jan;64(1):47-50. doi: 10.1111/j.1753-4887.2006.tb00173.x. Nutr Rev. 2006. PMID: 16491670 Review. - [The association of intestinal microbiota with obesity].
Morales P, Brignardello J, Gotteland M. Morales P, et al. Rev Med Chil. 2010 Aug;138(8):1020-7. Epub 2010 Nov 26. Rev Med Chil. 2010. PMID: 21140062 Review. Spanish.
Cited by
- Could the Gut Microbiota Serve as a Therapeutic Target in Ischemic Stroke?
Zhang J, Tang Q, Zhu L. Zhang J, et al. Evid Based Complement Alternat Med. 2021 Apr 17;2021:1391384. doi: 10.1155/2021/1391384. eCollection 2021. Evid Based Complement Alternat Med. 2021. PMID: 33959182 Free PMC article. Review. - Iron Fortification and Supplementation: Fighting Anemia of Chronic Diseases or Fueling Obesity?
El-Mallah CA, Beyh YS, Obeid OA. El-Mallah CA, et al. Curr Dev Nutr. 2021 Apr 7;5(4):nzab032. doi: 10.1093/cdn/nzab032. eCollection 2021 Apr. Curr Dev Nutr. 2021. PMID: 33959691 Free PMC article. - Microbiota conservation and BMI signatures in adult monozygotic twins.
Tims S, Derom C, Jonkers DM, Vlietinck R, Saris WH, Kleerebezem M, de Vos WM, Zoetendal EG. Tims S, et al. ISME J. 2013 Apr;7(4):707-17. doi: 10.1038/ismej.2012.146. Epub 2012 Nov 29. ISME J. 2013. PMID: 23190729 Free PMC article. - The human microbiome and its potential importance to pediatrics.
Johnson CL, Versalovic J. Johnson CL, et al. Pediatrics. 2012 May;129(5):950-60. doi: 10.1542/peds.2011-2736. Epub 2012 Apr 2. Pediatrics. 2012. PMID: 22473366 Free PMC article. Review. - Update on berberine in nonalcoholic Fatty liver disease.
Liu Y, Zhang L, Song H, Ji G. Liu Y, et al. Evid Based Complement Alternat Med. 2013;2013:308134. doi: 10.1155/2013/308134. Epub 2013 Jun 17. Evid Based Complement Alternat Med. 2013. PMID: 23843872 Free PMC article.
References
- Bouchard, C. (2000) N. Engl. J. Med. 343, 1888-1889. - PubMed
- Mokdad, A. H., Marks, J. S., Stroup, D. F. & Gerberding, J. L. (2004) J. Am. Med. Assoc. 291, 1238-1245. - PubMed
- Department of Health and Human Services (2001) The Surgeon General's Call to Action to Prevent and Decrease Overweight and Obesity (Public Health Service, Washington, DC).
- Friedman, J. M. (2004) Nat. Med. 10, 563-569. - PubMed
- Savage, D. C. (1977) Annu. Rev. Microbiol. 31, 107-133. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL058427/HL/NHLBI NIH HHS/United States
- R01 DK030292/DK/NIDDK NIH HHS/United States
- P30 DK056341/DK/NIDDK NIH HHS/United States
- DK 30292/DK/NIDDK NIH HHS/United States
- DK 56341/DK/NIDDK NIH HHS/United States
- R37 DK030292/DK/NIDDK NIH HHS/United States
- HL 58427/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases