Sole BCR-ABL inhibition is insufficient to eliminate all myeloproliferative disorder cell populations - PubMed (original) (raw)
Sole BCR-ABL inhibition is insufficient to eliminate all myeloproliferative disorder cell populations
S Wong et al. Proc Natl Acad Sci U S A. 2004.
Abstract
Protein kinase inhibitors can be effective in treating selected cancers, but most suppress several kinases. Imatinib mesylate has been useful in the treatment of Philadelphia chromosome-positive chronic myelogenous leukemia and B cell acute lymphoblastic leukemia through the inhibition of BCR-ABL tyrosine kinase activity. Imatinib mesylate has also been shown to inhibit KIT, ARG, and platelet-derived growth factor receptors alpha and beta, and potentially other tyrosine kinases. We have produced a mutant allele of BCR-ABL (T315A) that is uniquely inhibitable by the small molecule 4-amino-1-tert-butyl-3-(1-naphthyl)pyrazolo[3,4-d]pyrimidine and used it to demonstrate that sole suppression of BCR-ABL activity was insufficient to eliminate BCR-ABL(+) KIT(+)-expressing immature murine myeloid leukemic cells. In contrast, imatinib mesylate effectively eliminated BCR-ABL(+) KIT(+)-expressing leukemic cells. In the cellular context of mature myeloid cells and Pro/Pre B cells that do not express KIT, monospecific BCR-ABL inhibition was quantitatively as effective as imatinib mesylate in suppressing cell growth and inducing apoptosis. These results suggest that the therapeutic effectiveness of small molecule drugs such as imatinib mesylate could be due to the inhibitor's ability to suppress protein kinases in addition to the dominant target.
Figures
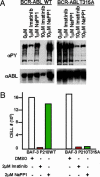
Fig. 1.
NaPP1 selectively suppresses BCR-ABL T315A-induced tyrosine phosphorylation and cytokine-independent growth without affecting BCR-ABL WT. (A) Concentrations (1 and 10 μM) of NaPP1 and imatinib mesylate were added to BCR-ABL T315A and BCR-ABL WT expressing BaF3 cells in the presence of 1% WeHI IL-3 containing medium, and total levels of tyrosine phosphorylation and BCR-ABL expression were measured 24 h after inhibitor addition as described (26). Equivalent volumes of DMSO were added to control cells. (B) Concentrations (2 μM) of NaPP1 and imatinib mesylate were added to BCR-ABL T315A and BCR-ABL WT expressing BaF3 cells in the absence of WeHI IL-3 containing medium every 24 h, and cell counts as measured by trypan blue exclusion were obtained 48 h after inhibitor addition. One set of data from a representative experiment repeated three times is shown.
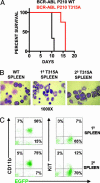
Fig. 2.
BCR-ABL T315A generates a transplantable MPD similar to BCR-ABL WT. (A) Survival curves of mice transplanted with BCR-ABL T315A or BCR-ABL WT cells. Generation of MPD was as described (27). Cell morphology (B) and cell surface expression (C) of CD11b and KIT in leukemic cells from mice transplanted BCR-ABL T315A expressing cells were conducted as described (–28). Similar patterns of expression were observed for BCR-ABL WT cell populations. Slides were photographed at ×1,000. Cytospins and flourescence-activated cell sorter plots represent an animal from each group, and similar results were obtained in all leukemic mice from each group.
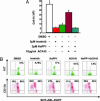
Fig. 3.
Imatinib mesylate eliminates a wider range of BCR-ABL T315A-expressing MPD cells than NaPP1. (A) Inhibitor concentrations as shown were added to BCR-ABL T315A-expressing MPD cells as described in Materials and Methods, and cell counts as measured by trypan blue exclusion were obtained 48 h after inhibitor addition. (B) Inhibitor concentrations as shown in A were added to BCR-ABL T315A-expressing MPD cells, and EGFP, KIT and CD11b levels were measured 48 h after inhibitor addition as described (–28). Two separate experiments were conducted in duplicate with similar results.
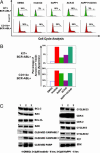
Fig. 4.
Simultaneous KIT and BCR-ABL inhibition has a synergistic effect in cell cycle suppression and an additive effect in apoptotic induction of KIT-positive MPD cells. (A) NaPP1 (2 μM), imatinib mesylate (2 μM), and ACK45 (10 μg/ml) inhibitor concentrations were added in the combinations shown to BCR-ABL T315A-expressing MPD cells every 24 h, and cell cycle analyses were conducted 48 h after inhibitor addition as described in Materials and Methods. (B) Inhibitor concentrations as shown in A were added in combinations shown to BCR-ABL T315A-expressing MPD cells every 24 h, and levels of annexin V staining were measured 48 h after inhibitor addition as described (26). (C) Concentrations of NaPP1 (2 μM) and imatinib mesylate (2 μM) were added to BCR-ABL T315A-expressing MPD cells, and levels of examined proteins were measured 6 h after inhibitor addition as described in Materials and Methods. Two separate experiments were conducted in duplicate with similar results.
Fig. 5.
In the cellular context of Pro/Pre B cells, NaPP1 is quantitatively and qualitatively as effective as imatinib mesylate in suppressing BCR-ABL-induced cell expansion. Generation of B-ALL was as described in Materials and Methods. (A) Flow cytometry analysis of EGFP, BP-1, and B220 expression was as described (–28). (B) NaPP1 (2 μM), imatinib mesylate (2 μM), and ACK45 (10 μg/ml) inhibitor concentrations were added every 24 h in combinations shown to BCR-ABL T315A-expressing Pro/Pre B cells, and viable cell counts as measured by trypan blue exclusion were obtained 48 h after inhibitor addition. BCR-ABL T315A-expressing Pro/Pre B cells were maintained as described (28). (C) Inhibitor concentrations as shown in B were added, and levels of annexin V staining were measured 48 h after inhibitor addition as described in Materials and Methods and ref. . (D) Inhibitor concentrations as described in B and DNA content for cell cycle analysis measured as described in Materials and Methods. (E) Concentrations of NaPP1 (2 μM) and imatinib mesylate (2 μM) were added to BCR-ABL T315A-expressing Pro/Pre B cells, and levels of noted proteins were measured 6 h after inhibitor addition as described in Materials and Methods. Two separate experiments were conducted in duplicate with similar results.
Similar articles
- Efficacy of dual-specific Bcr-Abl and Src-family kinase inhibitors in cells sensitive and resistant to imatinib mesylate.
Tipping AJ, Baluch S, Barnes DJ, Veach DR, Clarkson BM, Bornmann WG, Mahon FX, Goldman JM, Melo JV. Tipping AJ, et al. Leukemia. 2004 Aug;18(8):1352-6. doi: 10.1038/sj.leu.2403416. Leukemia. 2004. PMID: 15201856 - In BCR-ABL-positive cells, STAT-5 tyrosine-phosphorylation integrates signals induced by imatinib mesylate and Ara-C.
Kindler T, Breitenbuecher F, Kasper S, Stevens T, Carius B, Gschaidmeier H, Huber C, Fischer T. Kindler T, et al. Leukemia. 2003 Jun;17(6):999-1009. doi: 10.1038/sj.leu.2402940. Leukemia. 2003. PMID: 12764361 - ARG tyrosine kinase activity is inhibited by STI571.
Okuda K, Weisberg E, Gilliland DG, Griffin JD. Okuda K, et al. Blood. 2001 Apr 15;97(8):2440-8. doi: 10.1182/blood.v97.8.2440. Blood. 2001. PMID: 11290609 - Imatinib targets other than bcr/abl and their clinical relevance in myeloid disorders.
Pardanani A, Tefferi A. Pardanani A, et al. Blood. 2004 Oct 1;104(7):1931-9. doi: 10.1182/blood-2004-01-0246. Epub 2004 May 27. Blood. 2004. PMID: 15166033 Review. - [Molecular targeted treatment--new treatment strategy for patients with chronic myeloid leukemia].
Usui N. Usui N. Rinsho Byori. 2004 Feb;52(2):136-44. Rinsho Byori. 2004. PMID: 15027317 Review. Japanese.
Cited by
- Subfamily-specific adaptations in the structures of two penicillin-binding proteins from Mycobacterium tuberculosis.
Prigozhin DM, Krieger IV, Huizar JP, Mavrici D, Waldo GS, Hung LW, Sacchettini JC, Terwilliger TC, Alber T. Prigozhin DM, et al. PLoS One. 2014 Dec 31;9(12):e116249. doi: 10.1371/journal.pone.0116249. eCollection 2014. PLoS One. 2014. PMID: 25551456 Free PMC article. - N-myristoylated c-Abl tyrosine kinase localizes to the endoplasmic reticulum upon binding to an allosteric inhibitor.
Choi Y, Seeliger MA, Panjarian SB, Kim H, Deng X, Sim T, Couch B, Koleske AJ, Smithgall TE, Gray NS. Choi Y, et al. J Biol Chem. 2009 Oct 16;284(42):29005-14. doi: 10.1074/jbc.M109.026633. Epub 2009 Aug 13. J Biol Chem. 2009. PMID: 19679652 Free PMC article. - Phenotypic drug discovery: recent successes, lessons learned and new directions.
Vincent F, Nueda A, Lee J, Schenone M, Prunotto M, Mercola M. Vincent F, et al. Nat Rev Drug Discov. 2022 Dec;21(12):899-914. doi: 10.1038/s41573-022-00472-w. Epub 2022 May 30. Nat Rev Drug Discov. 2022. PMID: 35637317 Free PMC article. Review. - Kinase-independent mechanisms of resistance of leukemia stem cells to tyrosine kinase inhibitors.
Ichim CV. Ichim CV. Stem Cells Transl Med. 2014 Apr;3(4):405-15. doi: 10.5966/sctm.2012-0159. Epub 2014 Mar 5. Stem Cells Transl Med. 2014. PMID: 24598782 Free PMC article. Review. - Selective regulation of lymphopoiesis and leukemogenesis by individual zinc fingers of Ikaros.
Schjerven H, McLaughlin J, Arenzana TL, Frietze S, Cheng D, Wadsworth SE, Lawson GW, Bensinger SJ, Farnham PJ, Witte ON, Smale ST. Schjerven H, et al. Nat Immunol. 2013 Oct;14(10):1073-83. doi: 10.1038/ni.2707. Epub 2013 Sep 8. Nat Immunol. 2013. PMID: 24013668 Free PMC article.
References
- Blume-Jensen, P. & Hunter, T. (2001) Nature 411, 355–365. - PubMed
- Gilliland, D. G. & Griffin, J. D. (2002) Blood 100, 1532–1542. - PubMed
- Wong, S. & Witte, O. N. (2004) Annu. Rev. Immunol. 22, 247–306. - PubMed
- Blencke, S., Ullrich, A. & Daub, H. (2003) J. Biol. Chem. 278, 15435–15440. - PubMed
- Nowell, P. C. & Hungerford, D. A. (1960) Science 132, 1497–1501.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous