Persistent expression of chemokine and chemokine receptor RNAs at primary and latent sites of herpes simplex virus 1 infection - PubMed (original) (raw)
Persistent expression of chemokine and chemokine receptor RNAs at primary and latent sites of herpes simplex virus 1 infection
W James Cook et al. Virol J. 2004.
Abstract
Inflammatory cytokines and infiltrating T cells are readily detected in herpes simplex virus (HSV) infected mouse cornea and trigeminal ganglia (TG) during the acute phase of infection, and certain cytokines continue to be expressed at lower levels in infected TG during the subsequent latent phase. Recent results have shown that HSV infection activates Toll-like receptor signaling. Thus, we hypothesized that chemokines may be broadly expressed at both primary sites and latent sites of HSV infection for prolonged periods of time. Real-time reverse transcriptase-polymrease chain reaction (RT-PCR) to quantify expression levels of transcripts encoding chemokines and their receptors in cornea and TG following corneal infection. RNAs encoding the inflammatory-type chemokine receptors CCR1, CCR2, CCR5, and CXCR3, which are highly expressed on activated T cells, macrophages and most immature dendritic cells (DC), and the more broadly expressed CCR7, were highly expressed and strongly induced in infected cornea and TG at 3 and 10 days postinfection (dpi). Elevated levels of these RNAs persisted in both cornea and TG during the latent phase at 30 dpi. RNAs for the broadly expressed CXCR4 receptor was induced at 30 dpi but less so at 3 and 10 dpi in both cornea and TG. Transcripts for CCR3 and CCR6, receptors that are not highly expressed on activated T cells or macrophages, also appeared to be induced during acute and latent phases; however, their very low expression levels were near the limit of our detection. RNAs encoding the CCR1 and CCR5 chemokine ligands MIP-1alpha, MIP-1beta and RANTES, and the CCR2 ligand MCP-1 were also strongly induced and persisted in cornea and TG during the latent phase. These and other recent results argue that HSV antigens or DNA can stimulate expression of chemokines, perhaps through activation of Toll-like receptors, for long periods of time at both primary and latent sites of HSV infection. These chemokines recruit activated T cells and other immune cells, including DC, that express chemokine receptors to primary and secondary sites of infection. Prolonged activation of chemokine expression could provide mechanistic explanations for certain aspects of HSV biology and pathogenesis.
Figures
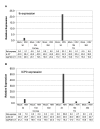
Figure 1
HSV tk and ICP0 RNA expression in mock and HSV-infected cornea and TG. RNA isolated from tissues harvested at 3, 10, or 30 days postinfection (d) was subjected to TaqMan RT-PCR analysis using HSV tk primers/probe (A) and HSV ICP0 primers/probe (B) as described in Materials and Methods. Mouse GAPDH RNA was measured in multiplex reactions, and used to calculate relative expression using the formula Rel Exp= 2-(ΔΔCT) × 1000 as described in Materials and Methods. Shown below the plots are relative expression values and the CT value measured for tk (A) and ICPO (B) in each sample. The ICP0 signal detected at 10 and 30 dpi in HSV-infected TG is likely due to LAT RNA as described in the text. Results shown are for one experiment (Experiment #1) in which the number of individual mouse tissues pooled were 10 for cornea and 6 for TG. Similar results were obtained in two additional experiments (Experiment #2 and Experiment #3), except for variation in detection of tk RNA in infected TG at 30 dpi as described in the text.
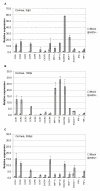
Figure 2
Relative levels of chemokine and chemokine receptor RNA expression in mock and HSV-infected cornea. Corneas were harvested at 3 (A), 10 (B), or 30 (C) days postinfection, and relative levels of expression were determined by TaqMan RT-PCR analysis as described in Fig. 1 and Materials and Methods. Results shown are the average of relative expression values determined using cDNA from two independent experiments, with each cDNA subjected to 2 or 3 separate measurements. Dashed bars represent ranges of individual values. Each cDNA was synthesized from RNA isolated from pooled corneas (5 mice) as described in Fig. 1 and Materials and Methods. The induction ratios (HSV+ vs. mock) for individual genes are tabulated in Table 3.
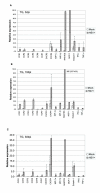
Figure 3
Relative levels of chemokine and chemokine receptor RNA expression in mock and HSV-infected TG. TG were harvested at 3 (A), 10 (B), or 30 (C) days postinfection, and RNA levels were determined by TaqMan RT-PCR analysis as described in Fig. 1, Fig. 2 and Materials and Methods. Results shown are the average of relative expression values determined using cDNA from three independent experiments, with each cDNA subjected to 2 or 3 separate measurements. Dashed bars represent ranges of individual values as described in Fig. 2. The induction ratios (HSV+ vs. mock) for individual genes are tabulated in Table 3.
Similar articles
- Dynamic expression of chemokines and the infiltration of inflammatory cells in the HSV-infected cornea and its associated tissues.
Araki-Sasaki K, Tanaka T, Ebisuno Y, Kanda H, Umemoto E, Hayashi K, Miyasaka M. Araki-Sasaki K, et al. Ocul Immunol Inflamm. 2006 Oct;14(5):257-66. doi: 10.1080/09273940600943581. Ocul Immunol Inflamm. 2006. PMID: 17056459 - Expression of herpes virus entry mediator (HVEM) in the cornea and trigeminal ganglia of normal and HSV-1 infected mice.
Kovacs SK, Tiwari V, Prandovszky E, Dosa S, Bacsa S, Valyi-Nagy K, Shukla D, Valyi-Nagy T. Kovacs SK, et al. Curr Eye Res. 2009 Oct;34(10):896-904. doi: 10.3109/02713680903184250. Curr Eye Res. 2009. PMID: 19895317 - CXCL10/CXCR3-Dependent Mobilization of Herpes Simplex Virus-Specific CD8+ TEM and CD8+ TRM Cells within Infected Tissues Allows Efficient Protection against Recurrent Herpesvirus Infection and Disease.
Srivastava R, Khan AA, Chilukuri S, Syed SA, Tran TT, Furness J, Bahraoui E, BenMohamed L. Srivastava R, et al. J Virol. 2017 Jun 26;91(14):e00278-17. doi: 10.1128/JVI.00278-17. Print 2017 Jul 15. J Virol. 2017. PMID: 28468883 Free PMC article. - [Battle with herpes for 37 years].
Shimomura Y. Shimomura Y. Nippon Ganka Gakkai Zasshi. 2015 Mar;119(3):145-66; discussion 167. Nippon Ganka Gakkai Zasshi. 2015. PMID: 25854108 Review. Japanese. - Herpes simplex virus latent infection in the nervous system.
Steiner I, Kennedy PG. Steiner I, et al. J Neurovirol. 1995 Mar;1(1):19-29. doi: 10.3109/13550289509111007. J Neurovirol. 1995. PMID: 9222339 Review.
Cited by
- Cytokines and chemokines: The vital role they play in herpes simplex virus mucosal immunology.
Smith JB, Herbert JJ, Truong NR, Cunningham AL. Smith JB, et al. Front Immunol. 2022 Sep 23;13:936235. doi: 10.3389/fimmu.2022.936235. eCollection 2022. Front Immunol. 2022. PMID: 36211447 Free PMC article. Review. - CXCL10 Chemokine: A Critical Player in RNA and DNA Viral Infections.
Elemam NM, Talaat IM, Maghazachi AA. Elemam NM, et al. Viruses. 2022 Nov 3;14(11):2445. doi: 10.3390/v14112445. Viruses. 2022. PMID: 36366543 Free PMC article. Review. - Inhibition of ataxia telangiectasia mutated (ATM) kinase suppresses herpes simplex virus type 1 (HSV-1) keratitis.
Alekseev O, Donovan K, Azizkhan-Clifford J. Alekseev O, et al. Invest Ophthalmol Vis Sci. 2014 Feb 3;55(2):706-15. doi: 10.1167/iovs.13-13461. Invest Ophthalmol Vis Sci. 2014. PMID: 24370835 Free PMC article. - Analysis of corneal inflammation induced by cauterisation in CCR2 and MCP-1 knockout mice.
Oshima T, Sonoda KH, Tsutsumi-Miyahara C, Qiao H, Hisatomi T, Nakao S, Hamano S, Egashira K, Charo IF, Ishibashi T. Oshima T, et al. Br J Ophthalmol. 2006 Feb;90(2):218-22. doi: 10.1136/bjo.2005.077875. Br J Ophthalmol. 2006. PMID: 16424537 Free PMC article. - Cytokine and chemokine regulation of sensory neuron function.
Miller RJ, Jung H, Bhangoo SK, White FA. Miller RJ, et al. Handb Exp Pharmacol. 2009;(194):417-49. doi: 10.1007/978-3-540-79090-7_12. Handb Exp Pharmacol. 2009. PMID: 19655114 Free PMC article. Review.
References
- Whitton LJ, Oldstone MBA. The immune response to viruses. In: Knipe D M and Howley P M, editor. Fields Virology, 4th ed. Philadelphia, PA, Lippincott, Williams and Wilkins; 2001. pp. 285–320.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous