Subcellular topography of neuronal Abeta peptide in APPxPS1 transgenic mice - PubMed (original) (raw)
Subcellular topography of neuronal Abeta peptide in APPxPS1 transgenic mice
Dominique Langui et al. Am J Pathol. 2004 Nov.
Abstract
In transgenic mice expressing human mutant beta-amyloid precursor protein (APP) and mutant presenilin-1 (PS1), Abeta antibodies labeled granules, about 1 microm in diameter, in the perikaryon of neurons clustered in the isocortex, hippocampus, amygdala, thalamus, and brainstem. The granules were present before the onset of Abeta deposits; their number increased up to 9 months and decreased in 15-month-old animals. They were immunostained by antibodies against Abeta 40, Abeta 42, and APP C-terminal region. In double immunofluorescence experiments, the intracellular Abeta co-localized with lysosome markers and less frequently with MG160, a Golgi marker. Abeta accumulation correlated with an increased volume of lysosomes and Golgi apparatus, while the volume of endoplasmic reticulum and early endosomes did not change. Some granules were immunolabeled with an antibody against flotillin-1, a raft marker. At electron microscopy, Abeta, APP-C terminal, cathepsin D, and flotillin-1 epitopes were found in the lumen of multivesicular bodies. This study shows that Abeta peptide and APP C-terminal region accumulate in multivesicular bodies containing lysosomal enzymes, while APP N-terminus is excluded from them. Multivesicular bodies could secondarily liberate their content in the extracellular space as suggested by the association of cathepsin D with Abeta peptide in the extracellular space.
Figures
Figure 1
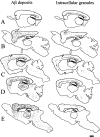
Maps of the extracellular Aβ deposits and Aβ-positive granules. The extracellular deposits of Aβ peptide (left) and the neurons containing Aβ-positive granules (right) were manually mapped using a dedicated software (Explora Nova, La Rochelle). Each dot is an Aβ deposit (left) or a granule containing neurone (right) A: 2 months; B: 5 months; C: 9 months; D: 11 months; E: 15 months. Bar, 1 mm.
Figure 2
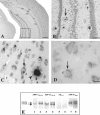
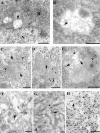
Immunoperoxidase Aβ8–17 immunostaining of Thy-1 double APP751SLxPS1M146L transgenic mouse (A, B, and C) and Thy-1 APP751SL single transgenic (D) mouse brain sections. The mice were 9 months (A, B, and C) and 5 months old (D). A: Distribution of Aβ immunoreactive deposits. Or, stratum oriens; Pyr, stratum pyramidale; Rad, stratum radiatum. The rectangle in A is enlarged in B. B: Dentate gyrus corresponding to rectangle in A, numerous plaques are visible (the arrow points to one of them). C: Intraneuronal granules (arrow) close to a plaque (arrowhead) in APPxPS1 transgenic animal. D: Intraneuronal granules (arrow) in an APP single transgenic animal. Bars: A, 0,5 mm; B, 50 μm; C, 15 μm; D, 10 μm. E: Brain homogenates from one wild-type mouse (lanes 1, 3, 5, and 7) and one Thy-1 double APP751SLxPS1M146L transgenic mouse (lanes 2, 4, 6, and 8) were analyzed by immunoblot using antibodies against APP Cter 705–751 (lanes 1 and 2), APP Cter 737–751 (lanes 3 and 4), Aβ8–17 (lanes 5 and 6), and APP Nter66–81 (lanes 7 and 8). Two bands (approximate molecular weight, 120 and 110 kd band, arrows) were detected by the antibodies to APP Cter and the antibody to APP Nter (22C11). These bands correspond to the full-length APP. The 120 kd visible in the transgenic animal corresponds to the human APP transgene. The antibody against Aβ8–17 did not cross-react with the full-length APP, either native or transgenic (lanes 5 and 6).
Figure 3
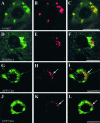
Double immunofluorescence examined with laser confocal microscope. Labeling with the Aβ peptide antibody is shown in red. Labeling with the other antibodies is shown in green. See Table 2 for details concerning the antibodies. Lamp2: lysosomal-associated membrane protein 2. Flotillin-1: a marker of raft and of multivesicular bodies. APP Cter, C terminus of the amyloid precursor protein (antibody raised against APP705–751); APP Nter, N terminus of the amyloid precursor protein (antibody raised against APP66–81). In H and I, arrows point to intraneuronal granules that are immunolabeled with both anti-Aβ8–17 and APP Cter (APP705–751) antibodies. In K and L, arrows point to intraneuronal granules, which are only labeled with anti-Aβ antibody (Aβ1–40 polyclonal, Chemicon) and not with the APP Nter antibody. Bars: A to F, 5 μm; G to L, 10 μm.
Figure 4
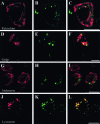
Double immunofluorescence examined with laser confocal microscope. Antibodies labeling organelles markers (A, D, G, and J) and Aβ8–17 (B, E, H, and K) are visualized respectively in red and in green. See Table 2 for details concerning the antibodies. The pictures on the right (C, F, I, and L) are merged images of the green and red signals; yellow indicates co-localization. Reticulum, endoplasmic reticulum labeled by Bip/GRP78; Golgi, Golgi apparatus labeled by MG160; Endosome, labeling by the antibody directed against EEA1 (early endosome autoantigen I). Lysosomes, organelles labeled by an antibody against cathepsin D, ie, lysosomes and multivesicular bodies having merged with lysosomes. Bars: A to C, 5 μm; D to L, 10 μm.
Figure 5
Proportion (%) of the total volume of intracellular Aβ peptide co-localized with the organelle marker. The Aβ antibody is the monoclonal antibody 6FD3, directed against amino acids 8–17 of the peptide (Dako). See Table 2 for details concerning the antibodies.
Figure 6
Electron microscopy. A: Conventional electron microscopy picture showing the appearance of intracellular granule (arrow); N, nucleus; R, endoplasmic reticulum. B to H: Immunoelectron microscopy. B: Labeling of an intraneuronal granule with anti-Aβ17–31 (E50,46). Gold particles are located in the lumenal vesicles (arrow). C: Double immunolabeling of intraneuronal granule with anti-Aβ17–31 (20-nm gold particle, arrowhead) and anti-flotillin (10-nm gold particle, arrow). D: Double immunolabeling of intraneuronal granule with anti-Aβ8–17 (10-nm gold particle, arrow) and anti-cathepsin D (20-nm gold particle, arrowhead). E: Double immunolabeling of a multivesicular body–lysosome with anti-APP Cter (20-nm gold particle, arrowhead) and anti-flotillin (10-nm gold particle, arrow). F: Labeling of an Aβ deposit with the anti-Aβ17–31 antibody decorating amyloid fibrils (arrowhead). The content of an extracellular vesicle is also labeled (arrow). (G) Labeling of Aβ deposit with the anti-cathepsin D antibody showing gold particles associated with amyloid fibrils (arrowhead) or between them (arrow). H: Double immunolabeling of an Aβ deposit with anti-Aβ17–31 (20-nm gold particle, arrowhead) and anti-flotillin (10-nm gold particle, arrow). Note that 10-nm gold particles are located in between the typical amyloid fibrils. Bars A to C and E to H, 400 nm; D, 250 nm.
Similar articles
- Presenilin 1 regulates the processing of beta-amyloid precursor protein C-terminal fragments and the generation of amyloid beta-protein in endoplasmic reticulum and Golgi.
Xia W, Zhang J, Ostaszewski BL, Kimberly WT, Seubert P, Koo EH, Shen J, Selkoe DJ. Xia W, et al. Biochemistry. 1998 Nov 24;37(47):16465-71. doi: 10.1021/bi9816195. Biochemistry. 1998. PMID: 9843412 - Neurodegenerative changes associated with beta-amyloid deposition in the brains of mice carrying mutant amyloid precursor protein and mutant presenilin-1 transgenes.
Kurt MA, Davies DC, Kidd M, Duff K, Rolph SC, Jennings KH, Howlett DR. Kurt MA, et al. Exp Neurol. 2001 Sep;171(1):59-71. doi: 10.1006/exnr.2001.7717. Exp Neurol. 2001. PMID: 11520121 - Mitochondria are devoid of amyloid β-protein (Aβ)-producing secretases: Evidence for unlikely occurrence within mitochondria of Aβ generation from amyloid precursor protein.
Mamada N, Tanokashira D, Ishii K, Tamaoka A, Araki W. Mamada N, et al. Biochem Biophys Res Commun. 2017 Apr 29;486(2):321-328. doi: 10.1016/j.bbrc.2017.03.035. Epub 2017 Mar 14. Biochem Biophys Res Commun. 2017. PMID: 28302486 - Altered levels and distribution of IGF-II/M6P receptor and lysosomal enzymes in mutant APP and APP + PS1 transgenic mouse brains.
Amritraj A, Hawkes C, Phinney AL, Mount HT, Scott CD, Westaway D, Kar S. Amritraj A, et al. Neurobiol Aging. 2009 Jan;30(1):54-70. doi: 10.1016/j.neurobiolaging.2007.05.004. Epub 2007 Jun 11. Neurobiol Aging. 2009. PMID: 17561313 - Aging increased amyloid peptide and caused amyloid plaques in brain of old APP/V717I transgenic mice by a different mechanism than mutant presenilin1.
Dewachter I, Van Dorpe J, Smeijers L, Gilis M, Kuipéri C, Laenen I, Caluwaerts N, Moechars D, Checler F, Vanderstichele H, Van Leuven F. Dewachter I, et al. J Neurosci. 2000 Sep 1;20(17):6452-8. doi: 10.1523/JNEUROSCI.20-17-06452.2000. J Neurosci. 2000. PMID: 10964951 Free PMC article.
Cited by
- The exosome secretory pathway transports amyloid precursor protein carboxyl-terminal fragments from the cell into the brain extracellular space.
Perez-Gonzalez R, Gauthier SA, Kumar A, Levy E. Perez-Gonzalez R, et al. J Biol Chem. 2012 Dec 14;287(51):43108-15. doi: 10.1074/jbc.M112.404467. Epub 2012 Nov 5. J Biol Chem. 2012. PMID: 23129776 Free PMC article. - PAT1 inversely regulates the surface Amyloid Precursor Protein level in mouse primary neurons.
Dilsizoglu Senol A, Tagliafierro L, Huguet L, Gorisse-Hussonnois L, Chasseigneaux S, Allinquant B. Dilsizoglu Senol A, et al. BMC Neurosci. 2015 Mar 7;16:10. doi: 10.1186/s12868-015-0152-8. BMC Neurosci. 2015. PMID: 25880931 Free PMC article. - Prostaglandin E2 stimulates the production of amyloid-beta peptides through internalization of the EP4 receptor.
Hoshino T, Namba T, Takehara M, Nakaya T, Sugimoto Y, Araki W, Narumiya S, Suzuki T, Mizushima T. Hoshino T, et al. J Biol Chem. 2009 Jul 3;284(27):18493-502. doi: 10.1074/jbc.M109.003269. Epub 2009 Apr 30. J Biol Chem. 2009. PMID: 19407341 Free PMC article. - Co-localization and distribution of cerebral APP and SP1 and its relationship to amyloidogenesis.
Brock B, Basha R, DiPalma K, Anderson A, Harry GJ, Rice DC, Maloney B, Lahiri DK, Zawia NH. Brock B, et al. J Alzheimers Dis. 2008 Feb;13(1):71-80. doi: 10.3233/jad-2008-13108. J Alzheimers Dis. 2008. PMID: 18334759 Free PMC article. - The polarity protein Par3 regulates APP trafficking and processing through the endocytic adaptor protein Numb.
Sun M, Asghar SZ, Zhang H. Sun M, et al. Neurobiol Dis. 2016 Sep;93:1-11. doi: 10.1016/j.nbd.2016.03.022. Epub 2016 Apr 9. Neurobiol Dis. 2016. PMID: 27072891 Free PMC article.
References
- Glenner GG, Wong CW. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–890. - PubMed
- Kang J, Lemaire HG, Unterbeck A, Salbaum JM, Masters CL, Grzeschik KH, Multhaup G, Beyreuther K, Muller-Hill B. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987;325:733–736. - PubMed
- Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. - PubMed
- Gouras GK, Xu H, Jovanovic JN, Buxbaum JD, Wang R, Greengard P, Relkin NR, Gandy S. Generation and regulation of beta-amyloid peptide variants by neurons. J Neurochem. 1998;71:1920–1925. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources