Induction of inflammatory mediators and microglial activation in mice transgenic for mutant human P301S tau protein - PubMed (original) (raw)
Induction of inflammatory mediators and microglial activation in mice transgenic for mutant human P301S tau protein
Arianna Bellucci et al. Am J Pathol. 2004 Nov.
Abstract
Mice transgenic for human P301S tau protein exhibit many characteristics of the human tauopathies, including the formation of abundant filaments made of hyperphosphorylated tau protein and neurodegeneration leading to nerve cell loss. At 5 months of age, the pathological changes are most marked in brainstem and spinal cord. Here we show that these changes are accompanied by marked neuroinflammation. Many tau-positive nerve cells in brainstem and spinal cord were strongly immunoreactive for interleukin-1beta and cyclooxygenase-2, indicating induction and overproduction of proinflammatory cytokines and enzymes. In parallel, numerous activated microglial cells were present throughout brain and spinal cord of transgenic mice, where they concentrated around tau-positive nerve cells. These findings suggest that inflammation may play a significant role in the events leading to neurodegeneration in the tauopathies and that anti-inflammatory compounds may have therapeutic potential.
Figures
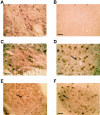
Figure 1
IL-1β immunoreactivity in brainstem and spinal cord of human P301S tau transgenic mice and age-matched controls. A and B: Brainstem of 5-month-old transgenic (A) and control (B) mice. C and D: Spinal cords of 5-month-old transgenic (C) and control (D) mice. Note the strong staining of some nerve cell bodies and processes (arrows in C). Scale bars: 150 μm (B, also representative for A); 71 μm (D, also representative for C).
Figure 2
COX-2 immunoreactivity in brain and spinal cord of human P301S tau transgenic mice. Strongly stained nerve cells and processes (arrows) in brainstem (A), spinal cord (C), cerebral cortex (D), hippocampus (E), and cerebellum (F) of 5-month-old human P301S tau transgenic mice. B: COX-2 immunoreactivity in brainstem of an age-matched control mouse. Note the strong staining of some nerve cell bodies and processes in A, C, D–F. Scale bars: 150 μm (B, also representative for A); 85 μm (C); 80 μm (D); 170 μm (E); 70 μm (F).
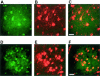
Figure 3
Double-labeling immunofluorescence staining for IL-1β (green) and tau phosphorylated at S202 and T205 (antibody AT8) (red) in brainstem and spinal cord of human P301S tau transgenic mice. A and B: Brainstem stained for IL-1β (A) and phosphorylated tau (B). C: Merged images shown in A and B. D and E: Spinal cord stained for IL-1β (D) and phosphorylated tau (E). F: Merged images shown in D and E. Co-localization is indicated by the yellow color. Scale bars: 60 μm (C, also representative for A, B); 125 μm (F, also representative for D, E).
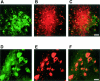
Figure 4
Double-labeling immunofluorescence staining for COX-2 (green) and tau phosphorylated at S202 and T205 (antibody AT8) (red) in brainstem and spinal cord of human P301S tau transgenic mice. A and B: Brainstem stained for COX-2 (A) and phosphorylated tau (B). C: Merged images shown in A and B. D and E: Spinal cord stained for COX-2 (D) and phosphorylated tau (E). F: Merged images shown in D and E. Co-localization is indicated by the yellow color. Scale bars: 45 μm (C, also representative for A, B); 40 μm (F, also representative for D, E).
Figure 5
Immunoblotting for IL-1β and COX-2 of spinal cord (left) and brainstem (right) from three human P301S tau transgenic mice and three age-matched controls. Immunoblotting for actin was used to ensure equal loading. Note the increased levels of proIL-1β, IL-1β and COX-2 in tissues from the transgenic mice.
Figure 6
Double-labeling immunostaining for OX-42 or OX-6 (brown) and tau phosphorylated at S422 (antibody AP422) (red) in brainstem and spinal cord of human P301S tau transgenic mice and age-matched controls. A, B, and E: Brainstem of transgenic (A, E) and control (B) mice. C, D, and F: Spinal cord of transgenic (C, F) and control mice (D). Sections A to E were stained for OX-42 and phosphorylated tau. Section F was stained for OX-6 and phosphorylated tau. Scale bars: 70 μm (B, also representative for A); 90 μm (D, also representative for C); 60 μm (E, F).
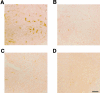
Figure 7
Major histocompatibility class II-immunoreactivity (antibody M5/114.15.2) in spinal cord of mice transgenic for human wild-type tau, human P301S tau, and age-matched controls. A and B: Five-month-old mouse transgenic for human P301S tau (A) and age-matched control (B). C and D: Fourteen-month-old mouse transgenic for human wild-type tau (C) and age-matched control (D). Scale bars: 50 μm (D, also representative for A–C). Note the staining of cells with the morphology of activated microglial cells in A.
Similar articles
- Early axonopathy preceding neurofibrillary tangles in mutant tau transgenic mice.
Leroy K, Bretteville A, Schindowski K, Gilissen E, Authelet M, De Decker R, Yilmaz Z, Buée L, Brion JP. Leroy K, et al. Am J Pathol. 2007 Sep;171(3):976-92. doi: 10.2353/ajpath.2007.070345. Epub 2007 Aug 9. Am J Pathol. 2007. PMID: 17690183 Free PMC article. - Cell-cycle markers in a transgenic mouse model of human tauopathy: increased levels of cyclin-dependent kinase inhibitors p21Cip1 and p27Kip1.
Delobel P, Lavenir I, Ghetti B, Holzer M, Goedert M. Delobel P, et al. Am J Pathol. 2006 Mar;168(3):878-87. doi: 10.2353/ajpath.2006.050540. Am J Pathol. 2006. PMID: 16507903 Free PMC article. - Using Experience Sampling Methodology to Capture Disclosure Opportunities for Autistic Adults.
Love AMA, Edwards C, Cai RY, Gibbs V. Love AMA, et al. Autism Adulthood. 2023 Dec 1;5(4):389-400. doi: 10.1089/aut.2022.0090. Epub 2023 Dec 12. Autism Adulthood. 2023. PMID: 38116059 Free PMC article. - Trends in Surgical and Nonsurgical Aesthetic Procedures: A 14-Year Analysis of the International Society of Aesthetic Plastic Surgery-ISAPS.
Triana L, Palacios Huatuco RM, Campilgio G, Liscano E. Triana L, et al. Aesthetic Plast Surg. 2024 Oct;48(20):4217-4227. doi: 10.1007/s00266-024-04260-2. Epub 2024 Aug 5. Aesthetic Plast Surg. 2024. PMID: 39103642 Review. - Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.
Salisbury L, Baraitser L. Salisbury L, et al. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
Cited by
- Microglial burden, activation and dystrophy patterns in frontotemporal lobar degeneration.
Woollacott IOC, Toomey CE, Strand C, Courtney R, Benson BC, Rohrer JD, Lashley T. Woollacott IOC, et al. J Neuroinflammation. 2020 Aug 10;17(1):234. doi: 10.1186/s12974-020-01907-0. J Neuroinflammation. 2020. PMID: 32778130 Free PMC article. - Redistribution of DAT/α-synuclein complexes visualized by "in situ" proximity ligation assay in transgenic mice modelling early Parkinson's disease.
Bellucci A, Navarria L, Falarti E, Zaltieri M, Bono F, Collo G, Spillantini MG, Missale C, Spano P. Bellucci A, et al. PLoS One. 2011;6(12):e27959. doi: 10.1371/journal.pone.0027959. Epub 2011 Dec 7. PLoS One. 2011. PMID: 22163275 Free PMC article. - Longitudinal TSPO expression in tau transgenic P301S mice predicts increased tau accumulation and deteriorated spatial learning.
Eckenweber F, Medina-Luque J, Blume T, Sacher C, Biechele G, Wind K, Deussing M, Briel N, Lindner S, Boening G, von Ungern-Sternberg B, Unterrainer M, Albert NL, Zwergal A, Levin J, Bartenstein P, Cumming P, Rominger A, Höglinger GU, Herms J, Brendel M. Eckenweber F, et al. J Neuroinflammation. 2020 Jul 13;17(1):208. doi: 10.1186/s12974-020-01883-5. J Neuroinflammation. 2020. PMID: 32660586 Free PMC article. - Differential effect of an evolving amyloid and tau pathology on brain phospholipids and bioactive lipid mediators in rat models of Alzheimer-like pathology.
Do Carmo S, Kautzmann MI, Bhattacharjee S, Jun B, Steinberg C, Emmerson JT, Malcolm JC, Bonomo Q, Bazan NG, Cuello AC. Do Carmo S, et al. J Neuroinflammation. 2024 Jul 30;21(1):185. doi: 10.1186/s12974-024-03184-7. J Neuroinflammation. 2024. PMID: 39080670 Free PMC article. - Hyperphosphorylated Tau Inflicts Intracellular Stress Responses that Are Mitigated by Apomorphine.
Song Z, Wang KW, Hagar HC, Chen HR, Kuan CY, Zhang K, Kuo MH. Song Z, et al. Mol Neurobiol. 2024 May;61(5):2653-2671. doi: 10.1007/s12035-023-03689-x. Epub 2023 Nov 3. Mol Neurobiol. 2024. PMID: 37919601 Free PMC article.
References
- Goedert M, Spillantini MG, Davies SW. Filamentous nerve cell inclusions in neurodegenerative diseases. Curr Opin Neurobiol. 1998;8:619–632. - PubMed
- Lee VMY, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–1159. - PubMed
- Poorkaj P, Bird TD, Wijsman E, Nemens E, Garruto RM, Anderson L, Andreadis A, Wiederholt WC, Raskind M, Schellenberg GD. Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann Neurol. 1998;43:815–825. - PubMed
- Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, Hackett J, Adamson J, Lincoln S, Dickson D, Davies P, Petersen RC, Stevens M, de Graaff E, Wauters E, van Baren J, Hillebrand M, Joosse M, Kwon JM, Nowotny P, Che LK, Norton J, Morris JC, Reed LA, Trojanowski JQ, Basun H, Lannfelt L, Neystat M, Fahn S, Dark F, Tannenberg T, Dodd PR, Hayward N, Kwok JBJ, Schofield PR, Andreadis A, Snowden J, Craufurd D, Neary D, Owen F, Oostra BA, Hardy J, Goate A, van Swieten J, Mann D, Lynch T, Heutink P. Association of missense and 5′-splice-site-mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials