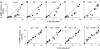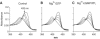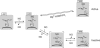NO activation of guanylyl cyclase - PubMed (original) (raw)
NO activation of guanylyl cyclase
Michael Russwurm et al. EMBO J. 2004.
Abstract
Nitric oxide (NO)-sensitive guanylyl-cyclase (GC) is the most important receptor for the signaling molecule NO. Activation of the enzyme is brought about by binding of NO to the prosthetic heme group. By monitoring NO-binding and catalytic activity simultaneously, we show that NO activates GC only if the reaction products of the enzyme are present. NO-binding in the absence of the products did not activate the enzyme, but yielded a nonactivated species with the spectral characteristics of the active form. Conversion of the nonactivated into the activated conformation of the enzyme required the simultaneous presence of NO and the reaction products. Furthermore, the products magnesium/cGMP/pyrophosphate promoted the release of the histidine-iron bond during NO-binding, indicating reciprocal communication of the catalytic and ligand-binding domains. Based on these observations, we present a model that proposes two NO-bound states of the enzyme: an active state formed in the presence of the products and a nonactivated state. The model not only covers the data reported here but also consolidates results from previous studies on NO-binding and dissociation/deactivation of GC.
Figures
Figure 1
Model for sequential binding of NO to GC. NO-binding first results in a 420 nm six-coordinated state that converts in an NO-dependent manner to the final 399 nm species via a proposed dinitrosyl-heme. The site of the heme to which NO is bound in the final 399 nm state is not identified. For further explanation, see text.
Figure 2
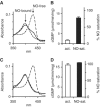
NO saturation and enzymatic activity of GC after removal of free NO. GC (90 μg, 6 μM) was saturated with NO by incubation with PROLI-NO (12.5 μM). Subsequently, free NO was removed on a desalting column. Spectra and activity of the eluted enzyme were measured simultaneously without or after addition of an excess of PROLI-NO (20 μM). (A) Representative spectrum of the eluted GC (solid line). For comparison, the NO-saturated, NO-free spectra (dashed lines) and a calculated spectrum of 80% NO-saturated GC (dotted line, hidden by the measured spectrum) are also shown. (B) Statistics of cGMP-forming activity of GC (open bar) and NO saturation (black) after removal of free NO. (C) Representative spectrum recorded after addition of PROLI-NO (20 μM) to the eluted GC. (D) Statistics of cGMP-forming activity of GC (open bar) and NO saturation (black) after addition of PROLI-NO (20 μM) to the eluted enzyme. Data in (B) and (D) are means±s.d. of three independent experiments.
Figure 3
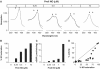
cGMP-forming activity and NO saturation of GC evoked by substoichiometric PROLI-NO. GC (6 μg, 0.4 μM) was preincubated with increasing PROLI-NO concentrations as indicated; spectra and enzymatic activity were recorded simultaneously. (A) Representative UV/vis spectra (solid lines) of GC preincubated with the indicated PROLI-NO concentrations were fitted with calculated spectra (dotted lines) to determine NO saturation of the enzyme. The position of the absorption maxima of the NO-free and the NO-bound enzyme are indicated by the arrows at 431 and 399 nm, respectively. (B) NO-saturation values of GC of the representative experiment depicted in (A) were obtained as described in Materials and methods section. (C) cGMP-forming activities of the samples depicted in (A) were determined in 1 min assays. (D) Relative activities were plotted versus relative NO-saturation values of _n_=28 simultaneous measurements of GC preincubated with increasing PROLI-NO concentrations; the NO saturation and activity of each sample is depicted as a black circle. The expected linear correlation is indicated by the line.
Figure 4
cGMP-forming activity and NO saturation of GC after preincubation with PROLI-NO in the presence of Mg2+ and PPi. GC (6 μg, 0.4 μM) was preincubated in the presence of Mg2+ and PPi (15 and 0.6 mM, respectively) with increasing PROLI-NO concentrations as indicated. Spectra and activity of the enzyme were determined simultaneously. (A) Representative spectra (solid lines) obtained after NO preincubation were compared to calculated spectra (dotted lines) to measure NO saturation of the enzyme. The position of the absorption maxima of the NO-free and the NO-bound enzyme are indicated by the arrows at 431 and 399 nm, respectively. (B) NO saturation of the samples shown in (A) was determined as described in Materials and methods section. (C) cGMP-forming activities of the samples depicted in (A) determined in a 1 min assay. (D) Relative activities were plotted versus relative NO-saturation values of _n_=21 samples; the NO saturation and activity of each sample is depicted as a black circle.
Figure 5
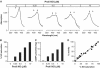
Correlation of cGMP-forming activity and NO saturation of GC in the presence of the substrate and reaction products of the enzyme. cGMP-forming activities and degree of NO saturations of GC (6 μg, 0.4 μM) were recorded simultaneously after preincubation with different amounts of PROLI-NO (0, 0.125, 0.25, 0.5, 1, 2, 10 μM) in the absence (A) and the presence of 15 mM MgCl2 (B), 1 mM cGMP (C), 0.6 mM PPi (D), or combinations thereof (E, G–I) as indicated, and in the presence of MgCl2/GTP (15/5 mM, respectively) (H). Each single pair of NO saturation and activity values is depicted as a black circle (A: _n_=28, B–I: _n_=21).
Figure 6
Monitoring sequential NO-binding in the presence of substrate and products. NO-binding to GC (1.5 μM) was followed at 4°C by recording UV/vis spectra after addition of DEA-NO (67 μM; the relatively high DEA-NO concentrations had to be used because of the slow decomposition of DEA-NO at 4°C). Spectra obtained in the absence (A) and the presence of 3 mM MgCl2 and 1 mM GTP (B), and 3 mM MgCl2 1 mM cGMP, 1 mM PPi (C) at 1, 14, 26, 38 and 62 s are shown.
Figure 7
Model for NO-binding and activation of NO-sensitive GC. NO-binding to the heme initially results in a six-coordinated state (420 nm) that immediately converts into the active state if the reaction products of the enzyme are present. In the absence of the enzyme's products, a nonactivated state is produced in an NO-dependent manner. Implications of the model are given in Discussion.
Similar articles
- Studying the structure and regulation of soluble guanylyl cyclase.
Koesling D. Koesling D. Methods. 1999 Dec;19(4):485-93. doi: 10.1006/meth.1999.0891. Methods. 1999. PMID: 10581148 Review. - Purification and characterization of NO-sensitive guanylyl cyclase.
Russwurm M, Koesling D. Russwurm M, et al. Methods Enzymol. 2005;396:492-501. doi: 10.1016/S0076-6879(05)96041-2. Methods Enzymol. 2005. PMID: 16291256 - Activation of soluble guanylate cyclase by carbon monoxide and nitric oxide: a mechanistic model.
Sharma VS, Magde D. Sharma VS, et al. Methods. 1999 Dec;19(4):494-505. doi: 10.1006/meth.1999.0892. Methods. 1999. PMID: 10581149 - Spectral and kinetic studies on the activation of soluble guanylate cyclase by nitric oxide.
Stone JR, Marletta MA. Stone JR, et al. Biochemistry. 1996 Jan 30;35(4):1093-9. doi: 10.1021/bi9519718. Biochemistry. 1996. PMID: 8573563 - Nitric oxide signaling: no longer simply on or off.
Cary SP, Winger JA, Derbyshire ER, Marletta MA. Cary SP, et al. Trends Biochem Sci. 2006 Apr;31(4):231-9. doi: 10.1016/j.tibs.2006.02.003. Epub 2006 Mar 10. Trends Biochem Sci. 2006. PMID: 16530415 Review.
Cited by
- Effect of oral organic nitrates on expression and activity of vascular soluble guanylyl cyclase.
Oppermann M, Dao VT, Suvorava T, Bas M, Kojda G. Oppermann M, et al. Br J Pharmacol. 2008 Oct;155(3):335-42. doi: 10.1038/bjp.2008.269. Epub 2008 Jun 30. Br J Pharmacol. 2008. PMID: 18587420 Free PMC article. - Structure and Activation of Soluble Guanylyl Cyclase, the Nitric Oxide Sensor.
Montfort WR, Wales JA, Weichsel A. Montfort WR, et al. Antioxid Redox Signal. 2017 Jan 20;26(3):107-121. doi: 10.1089/ars.2016.6693. Epub 2016 Apr 26. Antioxid Redox Signal. 2017. PMID: 26979942 Free PMC article. Review. - The Influence of Nitric Oxide on Soluble Guanylate Cyclase Regulation by Nucleotides: ROLE OF THE PSEUDOSYMMETRIC SITE.
Sürmeli NB, Müskens FM, Marletta MA. Sürmeli NB, et al. J Biol Chem. 2015 Jun 19;290(25):15570-15580. doi: 10.1074/jbc.M115.641431. Epub 2015 Apr 23. J Biol Chem. 2015. PMID: 25907555 Free PMC article. - The evolution of small molecule enzyme activators.
Dow LF, Case AM, Paustian MP, Pinkerton BR, Simeon P, Trippier PC. Dow LF, et al. RSC Med Chem. 2023 Sep 22;14(11):2206-2230. doi: 10.1039/d3md00399j. eCollection 2023 Nov 15. RSC Med Chem. 2023. PMID: 37974956 Free PMC article. Review. - Tonic and acute nitric oxide signaling through soluble guanylate cyclase is mediated by nonheme nitric oxide, ATP, and GTP.
Cary SP, Winger JA, Marletta MA. Cary SP, et al. Proc Natl Acad Sci U S A. 2005 Sep 13;102(37):13064-9. doi: 10.1073/pnas.0506289102. Epub 2005 Aug 30. Proc Natl Acad Sci U S A. 2005. PMID: 16131543 Free PMC article.
References
- Bellamy TC, Garthwaite J (2001) Sub-second kinetics of the nitric oxide receptor, soluble guanylyl cyclase, in intact cerebellar cells. J Biol Chem 276: 4287–4292 - PubMed
- Brandish PE, Buechler W, Marletta MA (1998) Regeneration of the ferrous heme of soluble guanylate cyclase from the nitric oxide complex: acceleration by thiols and oxyhemoglobin. Biochemistry 37: 16898–16907 - PubMed
- Garthwaite J, Boulton CL (1995) Nitric oxide signaling in the central nervous system. Annu Rev Physiol 57: 683–706 - PubMed
- Humbert P, Niroomand F, Fischer G, Mayer B, Koesling D, Hinsch KD, Gausepohl H, Frank R, Schultz G, Böhme E (1990) Purification of soluble guanylyl cyclase from bovine lung by a new immunoaffinity chromatographic method. Eur J Biochem 190: 273–278 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous