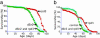Sir2 mediates longevity in the fly through a pathway related to calorie restriction - PubMed (original) (raw)
Sir2 mediates longevity in the fly through a pathway related to calorie restriction
Blanka Rogina et al. Proc Natl Acad Sci U S A. 2004.
Abstract
Calorie restriction can extend life span in a variety of species including mammals, flies, nematodes, and yeast. Despite the importance of this nearly universal effect, little is understood about the molecular mechanisms that mediate the life-span-extending effect of calorie restriction in metazoans. Sir2 is known to be involved in life span determination and calorie restriction in yeast mother cells. In nematodes increased Sir2 can extend life span, but a direct link to calorie restriction has not been demonstrated. We now report that Sir2 is directly involved in the calorie-restriction life-span-extending pathway in Drosophila. We demonstrate that an increase in Drosophila Sir2 (dSir2) extends life span, whereas a decrease in dSir2 blocks the life-span-extending effect of calorie reduction or rpd3 mutations. These data lead us to propose a genetic pathway by which calorie restriction extends life span and provides a framework for genetic and pharmacological studies of life span extension in metazoans.
Figures
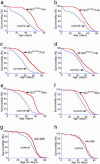
Fig. 1.
Increased expression of dSir2 extends life span in the fly. Life span extension is seen in female (a, c, e, and g) and male (b, d, f, and h) flies in which dSir2 expression is increased (red line) in a ubiquitous manner (a_–_d) or in the nervous system (e_–_h) compared with genetically matched controls (blue line). Survivorship curves for genetically matched controls and tubulin-GAL4 driver/dSir2EP2300 flies (a and b), tubulin-GAL4 driver/dSir2EP2384 flies (c and d), ELAV-GAL4 driver/dSir2EP2300 flies (e and f), and RU-486-ELAV-GeneSwitch driver/dSir2EP2300 flies (g and h). Genetically matched controls are flies derived from a second-generation cross of the offspring from the cross that generated the experimental flies in a_–_f. Controls in a_–_f are flies containing a mix of the chromosomes found in the experimental flies but which do not contain the GAL4 chromosome, the UAS-containing chromosome, or any balancer chromosome. Controls in g and h are flies from the same cohort fed only diluent and not RU-486. Each life span included at least 149 male and 159 female flies (16).
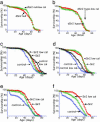
Fig. 2.
Calorie-reduction life span extension is mediated by dSir2. (a and b) The expected life span extension with calorie reduction was blocked by mutations in dSir2. Survivorship curves of dSir2-null [dSir24.5/dSir25.26 (29)] (a) and dSir2-hypomorphic (dSir2KG00871/dSir2KG00871) (b) female flies on normal (red filled triangles) and low-calorie (green open boxes) food. Male flies mutant for dSir2 also had no life span extension in response to caloric reduction. (c_–_f) The life span of long-lived dSir2-overexpressing flies was not further extended by calorie reduction. Survivorship curves of long-lived ELAV-GAL4/dSir2EP2300 (c and d) and tubulin-GAL4/dSir2EP2300 (e and f) male (c and e) and female (d and f) flies on normal (red filled triangles) and low-calorie (green open boxes) food and those of genetically matched controls (c and d) on normal (blue open circles) and low-calorie (black X's) food. Low-calorie food was as described in ref. and Materials and Methods. Each life span included at least 179 male and 171 female flies (16).
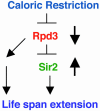
Fig. 3.
Calorie-restriction/Rpd3/Sir2 pathway for life span extension in the fly. In this model a stimulus of calorie reduction results in a decrease in Rpd3 (16). The reduction in Rpd3 activity decreases the inhibition of Sir2, causing an increase in Sir2 (16). The increase in Sir2, in conjunction with the decrease in Rpd3, effects life span extension.
Fig. 4.
Mutations in dSir2 block the life-span-extending effect of rpd3 mutants. The extension in life span seen with rpd3 mutation was prevented by mutations in dSir2. Flies labeled as “rpd3” are heterozygous for the _rpd3def24_-null allele of rpd3, whereas flies labeled as “dSir2 and rpd3” possess one copy of the life-span-extending _rpd3_-null mutation and one copy of either the _dSir217_-null (28) or _dSir2EP2300_-hypomorphic allele. (a) dSir217. Flies labeled “rpd3,” “dSir2 and rpd3,” and “control” were each backcrossed to a Canton-S background. (b) dSir2EP2300. Flies labeled “rpd3,” “dSir2 and rpd3,” and “control” were collected from the offspring of crosses between the _rpd3_-null allele, rpd3def24, and dSir2EP2300. Flies labeled “control” were derived from an additional cross to remove the rpd3 and dSir2EP2300 alleles and any balancer chromosomes. Each life span included at least 200 male and 200 female flies (16).
Similar articles
- Increased expression of Drosophila Sir2 extends life span in a dose-dependent manner.
Whitaker R, Faulkner S, Miyokawa R, Burhenn L, Henriksen M, Wood JG, Helfand SL. Whitaker R, et al. Aging (Albany NY). 2013 Sep;5(9):682-91. doi: 10.18632/aging.100599. Aging (Albany NY). 2013. PMID: 24036492 Free PMC article. - dSir2 and Dmp53 interact to mediate aspects of CR-dependent lifespan extension in D. melanogaster.
Bauer JH, Morris SN, Chang C, Flatt T, Wood JG, Helfand SL. Bauer JH, et al. Aging (Albany NY). 2009 Jan;1(1):38-48. doi: 10.18632/aging.100001. Aging (Albany NY). 2009. PMID: 19851477 Free PMC article. - HST2 mediates SIR2-independent life-span extension by calorie restriction.
Lamming DW, Latorre-Esteves M, Medvedik O, Wong SN, Tsang FA, Wang C, Lin SJ, Sinclair DA. Lamming DW, et al. Science. 2005 Sep 16;309(5742):1861-4. doi: 10.1126/science.1113611. Epub 2005 Jul 28. Science. 2005. PMID: 16051752 - dSir2 and longevity in Drosophila.
Frankel S, Ziafazeli T, Rogina B. Frankel S, et al. Exp Gerontol. 2011 May;46(5):391-6. doi: 10.1016/j.exger.2010.08.007. Epub 2010 Aug 20. Exp Gerontol. 2011. PMID: 20728527 Free PMC article. Review. - Calorie restriction and SIR2 genes--towards a mechanism.
Guarente L. Guarente L. Mech Ageing Dev. 2005 Sep;126(9):923-8. doi: 10.1016/j.mad.2005.03.013. Mech Ageing Dev. 2005. PMID: 15941577 Review.
Cited by
- NAD(+) Metabolism and the Control of Energy Homeostasis: A Balancing Act between Mitochondria and the Nucleus.
Cantó C, Menzies KJ, Auwerx J. Cantó C, et al. Cell Metab. 2015 Jul 7;22(1):31-53. doi: 10.1016/j.cmet.2015.05.023. Epub 2015 Jun 25. Cell Metab. 2015. PMID: 26118927 Free PMC article. Review. - Nature's Timepiece-Molecular Coordination of Metabolism and Its Impact on Aging.
Bednářová A, Kodrík D, Krishnan N. Bednářová A, et al. Int J Mol Sci. 2013 Jan 31;14(2):3026-49. doi: 10.3390/ijms14023026. Int J Mol Sci. 2013. PMID: 23434656 Free PMC article. - Age-associated molecular changes in the kidney in aged mice.
Lim JH, Kim EN, Kim MY, Chung S, Shin SJ, Kim HW, Yang CW, Kim YS, Chang YS, Park CW, Choi BS. Lim JH, et al. Oxid Med Cell Longev. 2012;2012:171383. doi: 10.1155/2012/171383. Epub 2012 Dec 30. Oxid Med Cell Longev. 2012. PMID: 23326623 Free PMC article. - Overexpression of SIRT1 protein in neurons protects against experimental autoimmune encephalomyelitis through activation of multiple SIRT1 targets.
Nimmagadda VK, Bever CT, Vattikunta NR, Talat S, Ahmad V, Nagalla NK, Trisler D, Judge SI, Royal W 3rd, Chandrasekaran K, Russell JW, Makar TK. Nimmagadda VK, et al. J Immunol. 2013 May 1;190(9):4595-607. doi: 10.4049/jimmunol.1202584. Epub 2013 Apr 1. J Immunol. 2013. PMID: 23547115 Free PMC article. - A Systematic Review of Antiaging Effects of 23 Traditional Chinese Medicines.
Wang L, Zuo X, Ouyang Z, Qiao P, Wang F. Wang L, et al. Evid Based Complement Alternat Med. 2021 May 15;2021:5591573. doi: 10.1155/2021/5591573. eCollection 2021. Evid Based Complement Alternat Med. 2021. PMID: 34055012 Free PMC article. Review.
References
- Masoro, E. J. (2000) Exp. Gerontol. 35, 299–305. - PubMed
- Lane, M. A., Mattison, J., Ingram, D. K. & Roth, G. S. (2002) Microsc. Res. Tech. 59, 335–338. - PubMed
- Mattison, J. A., Lane, M. A., Roth, G. S. & Ingram, D. K. (2003) Exp. Gerontol. 38, 35–46. - PubMed
- Wanagat, J., Allison, D. B. & Weindruch, R. (1999) Toxicol. Sci. 52, 35–40. - PubMed
- Weindruch, R. (1996) Toxicol. Pathol. 24, 742–745. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG023088/AG/NIA NIH HHS/United States
- ES 11463/ES/NIEHS NIH HHS/United States
- R37 AG016667/AG/NIA NIH HHS/United States
- RF1 AG024353/AG/NIA NIH HHS/United States
- R03 AG020816/AG/NIA NIH HHS/United States
- AG 20816/AG/NIA NIH HHS/United States
- AG 23088/AG/NIA NIH HHS/United States
- R01 AG016667/AG/NIA NIH HHS/United States
- AG 16667/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases