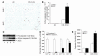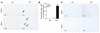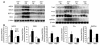Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice - PubMed (original) (raw)
Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice
Tirumalai Rangasamy et al. J Clin Invest. 2004 Nov.
Abstract
Although inflammation and protease/antiprotease imbalance have been postulated to be critical in cigarette smoke-induced (CS-induced) emphysema, oxidative stress has been suspected to play an important role in chronic obstructive pulmonary diseases. Susceptibility of the lung to oxidative injury, such as that originating from inhalation of CS, depends largely on its upregulation of antioxidant systems. Nuclear factor, erythroid-derived 2, like 2 (Nrf2) is a redox-sensitive basic leucine zipper protein transcription factor that is involved in the regulation of many detoxification and antioxidant genes. Disruption of the Nrf2 gene in mice led to earlier-onset and more extensive CS-induced emphysema than was found in wild-type littermates. Emphysema in Nrf2-deficient mice exposed to CS for 6 months was associated with more pronounced bronchoalveolar inflammation; with enhanced alveolar expression of 8-oxo-7,8-dihydro-2'-deoxyguanosine, a marker of oxidative stress; and with an increased number of apoptotic alveolar septal cells--predominantly endothelial and type II epithelial cells--as compared with wild-type mice. Microarray analysis identified the expression of nearly 50 Nrf2-dependent antioxidant and cytoprotective genes in the lung that may work in concert to counteract CS-induced oxidative stress and inflammation. The responsiveness of the Nrf2 pathway may act as a major determinant of susceptibility to tobacco smoke-induced emphysema by upregulating antioxidant defenses and decreasing lung inflammation and alveolar cell apoptosis.
Figures
Figure 1
Increased susceptibility of Nrf2–/– mice to CS-induced emphysema. Shown are H&E-stained lung sections from Nrf2+/+ and Nrf2–/– mice exposed to air alone (A and B, E and F, and I and J) and to CS (C and D, G and H, and K and L) at the indicated times. Sections from the air-exposed Nrf2+/+ and Nrf2–/– mice show normal alveolar structure (n = 5 per group). Lung sections from the CS-treated (6 months) Nrf2–/– mice show increased air space enlargement when compared with the lung sections from the CS-treated Nrf2+/+ mice. Original magnification, ×20.
Figure 2
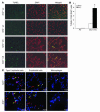
Cigarette smoke exposure causes lung cell apoptosis as assessed by TUNEL in Nrf2–/– lungs. (A) Lung sections (n = 5 per group) of room air– or CS-exposed (6 months) Nrf2+/+ or Nrf2–/– mice were subjected to TUNEL (left column) and DAPI stain (middle column). Merged images are shown in the right column. CS-exposed Nrf2–/– mice show abundant TUNEL-positive cells (arrows) in the alveolar septa. Magnification, ×20. (B) Quantification of TUNEL-positive cells (per 1,000 DAPI-stained cells). The number of TUNEL-positive cells was significantly higher in the CS-exposed Nrf2–/– mice as compared with their wild-type counterparts (*P – 0.05). Values represent mean ± SEM. (C) Identification of apoptotic (TUNEL-positive) type II epithelial cells (left column), endothelial cells (middle column), and alveolar macrophages (right column) in the lungs of CS-exposed (6 months) Nrf2+/+ and Nrf2–/– mice. Type II epithelial cells, endothelial cells, and alveolar macrophages were detected with anti-SpC, anti-CD34, and anti–Mac-3 antibodies, respectively, as outlined in Methods. Nuclei were detected with DAPI (blue). Shown are the merged images, with colocalization (yellow arrows) of cell-specific markers (cytoplasmic red signal) and apoptosis (nuclear green + blue DAPI signal, resulting in a lavender-like signal); non-apoptotic (TUNEL-negative) cells with positive cell specific marker (red signal) are highlighted with a red arrow. TUNEL-positive apoptotic cells lacking a cell-specific marker are highlighted by white arrowheads. The majority of TUNEL-positive cells consisted of endothelial and type II epithelial cells, whereas most alveolar macrophages were TUNEL negative. Scale bars: 5 μm.
Figure 3
CS treatment leads to activation of caspase-3 in Nrf2–/– lungs. (A) Active caspase-3 expression in lung sections from CS-exposed (6 months) Nrf2+/+ and Nrf2–/– mice. CS-exposed Nrf2 –/– mice show increased numbers of caspase-3–positive cells in the alveolar septa (n = 5 per group). Magnification, ×40. (B) Number of caspase-3–positive cells in the lungs of air- and CS-exposed mice. Caspase-3–positive cells were significantly higher in the lungs of CS-exposed Nrf2–/– mice. (C) Increased expression of the 18-kDa active form of caspase-3 in lungs of CS-exposed (6 months) Nrf2–/– mice (Western blot; lanes 1 and 3: air- and CS-exposed Nrf2+/+ mice, respectively; lanes 2 and 4: air- and CS-exposed Nrf2–/– mice, respectively). (D) Quantification of procaspase-3 and active caspase-3 obtained in Western blots of air- or CS-exposed Nrf2+/+ and Nrf2–/– lungs. Values are represented as mean ± SEM. (E) Caspase-3 activity in the lungs of air- or CS-exposed (6 months) Nrf2+/+ and Nrf2–/– mice. Caspase-3 activity was significantly higher in the lungs of CS-exposed Nrf2–/– mice than in the lungs of their wild-type counterparts (n = 3 per group). Values (relative fluorescence units [RFU]) are represented as mean ± SEM. *P – 0.05 vs. CS-exposed Nrf2+/+ mice.
Figure 4
Increased sensitivity of Nrf2–/– mice to oxidative stress after CS exposure. (A) Immunohistochemical staining for 8-oxo-dG in lung sections from the mice exposed to CS (6 months) (n = 5 per group). Lung sections from the CS-exposed Nrf2–/– mice show increased staining for 8-oxo-dG (indicated by arrows) when compared with lung sections from CS-exposed Nrf2+/+ mice and the respective air-exposed control mice. Magnification, ×40. (B) Quantification of 8-oxo-dG–positive alveolar septal cells in lungs after 6 months of CS exposure. The number of cells that reacted with anti–8-oxo-dG antibody was significantly higher in the lung tissues of the CS-exposed Nrf2–/– mice than in the lung tissues of the CS-exposed Nrf2+/+ mice and air-exposed control mice. Values (positive cells/mm alveolar length) represent mean ± SEM. *P – 0.05 vs. CS-exposed Nrf2+/+ mice. (C) Immunohistochemical staining with normal mouse-IgG1 antibody in lung sections from air- or CS-exposed Nrf2+/+ and Nrf2–/– mice. Magnification, ×40. Scale bars: 25 μm.
Figure 5
Increased inflammation in the lungs of CS-exposed Nrf2–/– mice. (A) Lavaged inflammatory cells from control and CS-exposed mice. The number of macrophages in BAL fluid collected from the CS-exposed (both 1.5 months and 6 months) Nrf2–/– mice was significantly higher than in the BAL fluid from CS-exposed Nrf2+/+ mice and the respective age-matched control mice. Values represent mean ± SEM (n = 8). PMNs, polymorphonuclear leukocytes. *P – 0.05 vs. control of the same genotype; P – 0.05 across the genotypes in CS-exposed group. (B) Immunohistochemical detection of macrophages (arrows) in lungs of Nrf2+/+ and Nrf2–/– mice exposed to CS for 6 months. Magnification, ×40. Scale bars: 25 μm. (C) Quantification of macrophages in lungs after 6 months of CS exposure. The lung sections from the CS-exposed Nrf2–/– mice showed significantly more macrophages than did those from wild-type counterparts exposed to CS (**P – 0.025). However, there was no significant difference in the number of alveolar macrophages between the air-exposed Nrf2+/+ and Nrf2–/– mice (P > 0.9).
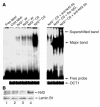
Figure 6
Activation of Nrf2 in CS-exposed Nrf2+/+ lungs. (A) EMSA to determine the DNA binding activity of Nrf2. For gel shift analysis, 10 μg of nuclear protein from the lungs of air-and CS-exposed mice was incubated with the labeled human NQO1 ARE sequence and analyzed on a 5% non-denaturing polyacrylamide gel. For supershift assays, the labeled NQO1 ARE was first incubated with 10 μg of nuclear extract and then with 4 μg of anti-Nrf2 antibody for 2 hours. Nuclear protein of Nrf2+/+ lungs showed increased binding to the ARE-containing sequence (lower arrow) after CS exposure, with a supershifted band caused by preincubation with anti-Nrf2 antibody, thus confirming the binding of Nrf2 to the ARE sequence (upper arrow). Ra–IgG1, rabbit IgG1. (B) Nuclear accumulation of Nrf2. Western blot analysis with anti-Nrf2 antibody showed the nuclear accumulation of the transcription factor Nrf2 in the lungs of Nrf2+/+ mice in response to CS exposure (lanes 1 and 3: air-exposed Nrf2–/– and Nrf2+/+ mice, respectively; lanes 2 and 4: CS-exposed Nrf2–/– and Nrf2+/+ mice, respectively; lamin B1 was used as the loading control). Western blot analysis was carried out 3 times with the nuclear proteins isolated from the lungs of 3 different air- or CS-exposed Nrf2+/+ and Nrf2–/– mice.
Figure 7
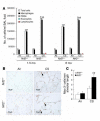
Validation of microarray data by Northern blot and enzyme assays. (A) Analysis of mRNA levels of NQO1, GCLm, GST-α1, HO-1, TrxR, Prx-1, GSR, and G6PDH in the lungs of Nrf2+/+ and Nrf2–/– mice exposed to either air or CS (n = 3 per group). (B) Effect of CS on the specific activities of selected enzymes in the lungs of Nrf2+/+ and Nrf2–/– mice. Values represent mean ± SE (n = 3 per group). *P – 0.05 vs. control of the same genotype.
Similar articles
- The transcriptome of Nrf2-/- mice provides evidence for impaired cell cycle progression in the development of cigarette smoke-induced emphysematous changes.
Gebel S, Diehl S, Pype J, Friedrichs B, Weiler H, Schüller J, Xu H, Taguchi K, Yamamoto M, Müller T. Gebel S, et al. Toxicol Sci. 2010 May;115(1):238-52. doi: 10.1093/toxsci/kfq039. Epub 2010 Feb 4. Toxicol Sci. 2010. PMID: 20133372 - Ursolic acid attenuates cigarette smoke-induced emphysema in rats by regulating PERK and Nrf2 pathways.
Lin L, Yin Y, Hou G, Han D, Kang J, Wang Q. Lin L, et al. Pulm Pharmacol Ther. 2017 Jun;44:111-121. doi: 10.1016/j.pupt.2017.03.014. Epub 2017 Mar 27. Pulm Pharmacol Ther. 2017. PMID: 28347799 - Nrf2-deficient mice are highly susceptible to cigarette smoke-induced emphysema.
Iizuka T, Ishii Y, Itoh K, Kiwamoto T, Kimura T, Matsuno Y, Morishima Y, Hegab AE, Homma S, Nomura A, Sakamoto T, Shimura M, Yoshida A, Yamamoto M, Sekizawa K. Iizuka T, et al. Genes Cells. 2005 Dec;10(12):1113-25. doi: 10.1111/j.1365-2443.2005.00905.x. Genes Cells. 2005. PMID: 16324149 - NRF2 targeting: a promising therapeutic strategy in chronic obstructive pulmonary disease.
Boutten A, Goven D, Artaud-Macari E, Boczkowski J, Bonay M. Boutten A, et al. Trends Mol Med. 2011 Jul;17(7):363-71. doi: 10.1016/j.molmed.2011.02.006. Epub 2011 Apr 1. Trends Mol Med. 2011. PMID: 21459041 Review. - Oxidative stress targets in pulmonary emphysema: focus on the Nrf2 pathway.
Boutten A, Goven D, Boczkowski J, Bonay M. Boutten A, et al. Expert Opin Ther Targets. 2010 Mar;14(3):329-46. doi: 10.1517/14728221003629750. Expert Opin Ther Targets. 2010. PMID: 20148719 Review.
Cited by
- Non-electrophilic NRF2 activators promote wound healing in human keratinocytes and diabetic mice and demonstrate selective downstream gene targeting.
Barakat M, Han C, Chen L, David BP, Shi J, Xu A, Skowron KJ, Johnson T, Woods RA, Ankireddy A, Reddy SP, Moore TW, DiPietro LA. Barakat M, et al. Sci Rep. 2024 Oct 24;14(1):25258. doi: 10.1038/s41598-024-75786-3. Sci Rep. 2024. PMID: 39448644 Free PMC article. - Immunogenetic and Environmental Factors in Age-Related Macular Disease.
Brodzka S, Baszyński J, Rektor K, Hołderna-Bona K, Stanek E, Kurhaluk N, Tkaczenko H, Malukiewicz G, Woźniak A, Kamiński P. Brodzka S, et al. Int J Mol Sci. 2024 Jun 14;25(12):6567. doi: 10.3390/ijms25126567. Int J Mol Sci. 2024. PMID: 38928273 Free PMC article. Review. - Role of CXCL5 in leukocyte recruitment to the lungs during secondhand smoke exposure.
Balamayooran G, Batra S, Cai S, Mei J, Worthen GS, Penn AL, Jeyaseelan S. Balamayooran G, et al. Am J Respir Cell Mol Biol. 2012 Jul;47(1):104-11. doi: 10.1165/rcmb.2011-0260OC. Epub 2012 Feb 23. Am J Respir Cell Mol Biol. 2012. PMID: 22362385 Free PMC article. - Nrf2-Mediated Dichotomy in the Vascular System: Mechanistic and Therapeutic Perspective.
Wu W, Hendrix A, Nair S, Cui T. Wu W, et al. Cells. 2022 Sep 28;11(19):3042. doi: 10.3390/cells11193042. Cells. 2022. PMID: 36231004 Free PMC article. Review. - Food-Derived Pharmacological Modulators of the Nrf2/ARE Pathway: Their Role in the Treatment of Diseases.
Zhao F, Ci X, Man X, Li J, Wei Z, Zhang S. Zhao F, et al. Molecules. 2021 Feb 15;26(4):1016. doi: 10.3390/molecules26041016. Molecules. 2021. PMID: 33671866 Free PMC article. Review.
References
- Petty TL. Definition, epidemiology, course, and prognosis of COPD. Clin. Cornerstone. 2003;5:1–10. - PubMed
- Viegi G, Scognamiglio A, Baldacci S, Pistelli F, Carrozzi L. Epidemiology of chronic obstructive pulmonary disease (COPD) Respiration. 2001;68:4–19. - PubMed
- Tuder RM, et al. Oxidative stress and apoptosis interact and cause emphysema due to vascular endothelial growth factor receptor blockade. Am. J. Respir. Cell Mol. Biol. 2003;29:88–97. - PubMed
- Eriksson S. Pulmonary emphysema and α1-antitrypsin deficiency. Acta Med. Scand. 1964;175:197–205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 66554/PHS HHS/United States
- R01 CA94076/CA/NCI NIH HHS/United States
- R01 CA094076/CA/NCI NIH HHS/United States
- P50 CA058184/CA/NCI NIH HHS/United States
- R01 HL105772/HL/NHLBI NIH HHS/United States
- P30 ES 03819/ES/NIEHS NIH HHS/United States
- P30 ES003819/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases