Ink4a/Arf expression is a biomarker of aging - PubMed (original) (raw)
Ink4a/Arf expression is a biomarker of aging
Janakiraman Krishnamurthy et al. J Clin Invest. 2004 Nov.
Abstract
The Ink4a/Arf locus encodes 2 tumor suppressor molecules, p16INK4a and Arf, which are principal mediators of cellular senescence. To study the links between senescence and aging in vivo, we examined Ink4a/Arf expression in rodent models of aging. We show that expression of p16INK4a and Arf markedly increases in almost all rodent tissues with advancing age, while there is little or no change in the expression of other related cell cycle inhibitors. The increase in expression is restricted to well-defined compartments within each organ studied and occurs in both epithelial and stromal cells of diverse lineages. The age-associated increase in expression of p16INK4a and Arf is attenuated in the kidney, ovary, and heart by caloric restriction, and this decrease correlates with diminished expression of an in vivo marker of senescence, as well as decreased pathology of those organs. Last, the age-related increase in Ink4a/Arf expression can be independently attributed to the expression of Ets-1, a known p16INK4a transcriptional activator, as well as unknown Ink4a/Arf coregulatory molecules. These data suggest that expression of the Ink4a/Arf tumor suppressor locus is a robust biomarker, and possible effector, of mammalian aging.
Figures
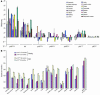
Figure 1
Expression of the Ink4a/Arf locus increases with aging. (A) Relative expression. The ratios (log2 scale) of the expression of cell cycle inhibitors – old (26 months)/young (2.5 months) – from 15 tissues is graphed ± SEM. Each estimate represents the mean of 8–32 quantitative RT-PCR reactions on independent RNA samples derived from 4–6 mice. *Minimum estimate of old/young ratio. (B) Absolute expression. The absolute copy number of p16INK4a and Arf mRNA molecules (log10 scale) per 90 ng total RNA RT-PCR from 15 tissues of young (2.5 months) and old (26 months) mice is graphed ± SEM. Murine embryo fibroblasts (MEFs) at early (P4) and late (P7) passage are shown for comparison. #Maximum estimated expression is indicated, as expression was below the level of detection.
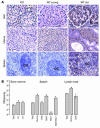
Figure 2
p16INK4a expression in specific compartments by immunohistochemistry and cell purification. (A) Immunoperoxidase staining performed on paraffin-embedded sections of germ-line _p16INK4a_-deficient (KO), WT young (3.5 months), and WT old (25 months) murine tissues using an anti-p16INK4a antibody. Positively staining cells demonstrate both nuclear and cytoplasmic expression. GC, germinal center. (B) Relative expression ratios (old/young, log2 scale) of p16INK4a in specific compartments (average purity >94% for all fractions) of bone marrow (lin–, 2%; lin+, 97%), spleen (B220+, 48%; Mac1+, 9%; B220–Mac1–, 22%), and lymph node. Asterisks indicate that p16INK4a expression was undetectable in these cell populations from young mice, and therefore a minimum estimate of the fold increase is shown.
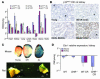
Figure 3
Effects of caloric restriction and GHR deficiency on gene expression and aging. (A) Relative expression ratios (old/young, log2 scale) of cell cycle inhibitors in 7 tissues derived from old (28 months) and young (3 months) AL or CR F344 rats. The relative ratios are graphed ± SEM. Each estimate represents the mean of 8–16 quantitative RT-PCR reactions on independent RNA samples derived from 4 rats. (B) Immunoperoxidase staining on paraffin-embedded kidney sections from young, old AL, and old CR F344 rats using an anti-p16INK4a antibody. G, glomeruli seen in cortical sections. (C) SA-β-gal staining in AL and CR mouse and rat kidney. C, renal cortex; M, renal medulla. Thin tissue slices were stained for mice, as opposed to small tissue wedges for rats. SA-β-gal activity is predominantly restricted to the renal cortex. (D) Relative expression ratios (old/young, log2 scale) of Ets-1 in kidneys derived from AL and CR rats and mice. Results from the kidneys from AL and CR mice with and without GHR deficiency are also shown. Each estimate represents the mean of 8–32 quantitative RT-PCR reactions on independent RNA samples derived from 8 mice or 4 rats.
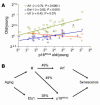
Figure 4
p16INK4a expression with aging strongly correlates with Arf and Ets-1 expression. (A) A scatter plot (log2 scale, both axes) of the ratios (old/young) of p16INK4a expression versus the expression ratios (old/young) of Arf, Ets-1, and Id1 seen in the corresponding tissue (n = 22–70 data pairs per gene from up to 15 tissues in both mouse and rat). Each ratio represents a mean value of multiple measurements per tissue as described in Methods. A best-fit line determined by linear regression is shown for each data series, with Pearson correlation coefficient and 2-tailed P value. No significant correlation was seen between Arf and Ets-1, or between Arf or p16INK4a and Bmi-1 (not shown). (B) Arrows show known or inferred transcriptional relationships, and numbers indicate the covariances (_r_2) for the linked elements as determined in A. As p16INK4a and Arf do not regulate one another, it seems reasonable to assume that an unknown coregulator (X) modulates the expression of both transcripts with aging, explaining their strong correlation (_r_2 = 48%). Furthermore, as Arf and Ets-1 do not covary, X and Ets-1 must be independent. X need not represent a single transcription factor: it may represent the combined activity of several genes (e.g., the PcG family members) or genes that affect other transcript properties, such as message stability. This model suggests that the majority (87%) of the variance in p16INK4a expression with aging in the analyzed tissues can be attributed to the activity of X and Ets-1.
Comment in
- p16 and ARF: activation of teenage proteins in old age.
Satyanarayana A, Rudolph KL. Satyanarayana A, et al. J Clin Invest. 2004 Nov;114(9):1237-40. doi: 10.1172/JCI23437. J Clin Invest. 2004. PMID: 15520854 Free PMC article. Review.
Similar articles
- The inhibitor of cyclin-dependent kinase 4a/alternative reading frame (INK4a/ARF) locus encoded proteins p16INK4a and p19ARF repress cyclin D1 transcription through distinct cis elements.
D'Amico M, Wu K, Fu M, Rao M, Albanese C, Russell RG, Lian H, Bregman D, White MA, Pestell RG. D'Amico M, et al. Cancer Res. 2004 Jun 15;64(12):4122-30. doi: 10.1158/0008-5472.CAN-03-2519. Cancer Res. 2004. PMID: 15205322 - p16 and ARF: activation of teenage proteins in old age.
Satyanarayana A, Rudolph KL. Satyanarayana A, et al. J Clin Invest. 2004 Nov;114(9):1237-40. doi: 10.1172/JCI23437. J Clin Invest. 2004. PMID: 15520854 Free PMC article. Review. - Recovery of function in osteoarthritic chondrocytes induced by p16INK4a-specific siRNA in vitro.
Zhou HW, Lou SQ, Zhang K. Zhou HW, et al. Rheumatology (Oxford). 2004 May;43(5):555-68. doi: 10.1093/rheumatology/keh127. Epub 2004 Mar 16. Rheumatology (Oxford). 2004. PMID: 15026580 - Ink4a/Arf links senescence and aging.
Sharpless NE. Sharpless NE. Exp Gerontol. 2004 Nov-Dec;39(11-12):1751-9. doi: 10.1016/j.exger.2004.06.025. Exp Gerontol. 2004. PMID: 15582292 Review. - Cdk4 disruption renders primary mouse cells resistant to oncogenic transformation, leading to Arf/p53-independent senescence.
Zou X, Ray D, Aziyu A, Christov K, Boiko AD, Gudkov AV, Kiyokawa H. Zou X, et al. Genes Dev. 2002 Nov 15;16(22):2923-34. doi: 10.1101/gad.1033002. Genes Dev. 2002. PMID: 12435633 Free PMC article.
Cited by
- Clinical significance of cell cycle inhibitors in hepatocellular carcinoma.
Matsuda Y, Wakai T, Kubota M, Takamura M, Yamagiwa S, Aoyagi Y, Osawa M, Fujimaki S, Sanpei A, Genda T, Ichida T. Matsuda Y, et al. Med Mol Morphol. 2013 Dec;46(4):185-92. doi: 10.1007/s00795-013-0047-7. Epub 2013 May 3. Med Mol Morphol. 2013. PMID: 23640750 Review. - An aged immune system drives senescence and ageing of solid organs.
Yousefzadeh MJ, Flores RR, Zhu Y, Schmiechen ZC, Brooks RW, Trussoni CE, Cui Y, Angelini L, Lee KA, McGowan SJ, Burrack AL, Wang D, Dong Q, Lu A, Sano T, O'Kelly RD, McGuckian CA, Kato JI, Bank MP, Wade EA, Pillai SPS, Klug J, Ladiges WC, Burd CE, Lewis SE, LaRusso NF, Vo NV, Wang Y, Kelley EE, Huard J, Stromnes IM, Robbins PD, Niedernhofer LJ. Yousefzadeh MJ, et al. Nature. 2021 Jun;594(7861):100-105. doi: 10.1038/s41586-021-03547-7. Epub 2021 May 12. Nature. 2021. PMID: 33981041 Free PMC article. - Multiparameter flow cytometric detection and quantification of senescent cells in vitro.
Adewoye AB, Tampakis D, Follenzi A, Stolzing A. Adewoye AB, et al. Biogerontology. 2020 Dec;21(6):773-786. doi: 10.1007/s10522-020-09893-9. Epub 2020 Aug 10. Biogerontology. 2020. PMID: 32776262 Free PMC article. - Cellular senescence is increased in airway smooth muscle cells of elderly persons with asthma.
Aghali A, Khalfaoui L, Lagnado AB, Drake LY, Teske JJ, Pabelick CM, Passos JF, Prakash YS. Aghali A, et al. Am J Physiol Lung Cell Mol Physiol. 2022 Nov 1;323(5):L558-L568. doi: 10.1152/ajplung.00146.2022. Epub 2022 Sep 27. Am J Physiol Lung Cell Mol Physiol. 2022. PMID: 36166734 Free PMC article. - Cellular senescence in age-related disorders.
Kaur J, Farr JN. Kaur J, et al. Transl Res. 2020 Dec;226:96-104. doi: 10.1016/j.trsl.2020.06.007. Epub 2020 Jun 20. Transl Res. 2020. PMID: 32569840 Free PMC article. Review.
References
- Guarente L, Kenyon C. Genetic pathways that regulate ageing in model organisms. Nature. 2000;408:255–262. - PubMed
- Campisi J. Cancer and ageing: rival demons? Nat. Rev. Cancer. 2003;3:339–349. - PubMed
- Sharpless NE, DePinho RA. p53: good cop/bad cop. Cell. 2002;110:9–12. - PubMed
- Artandi SE, et al. Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature. 2000;406:641–645. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous