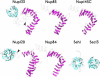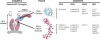Components of coated vesicles and nuclear pore complexes share a common molecular architecture - PubMed (original) (raw)
Components of coated vesicles and nuclear pore complexes share a common molecular architecture
Damien Devos et al. PLoS Biol. 2004 Dec.
Erratum in
- PLoS Biol. 2005 Feb;3(2):e80
Abstract
Numerous features distinguish prokaryotes from eukaryotes, chief among which are the distinctive internal membrane systems of eukaryotic cells. These membrane systems form elaborate compartments and vesicular trafficking pathways, and sequester the chromatin within the nuclear envelope. The nuclear pore complex is the portal that specifically mediates macromolecular trafficking across the nuclear envelope. Although it is generally understood that these internal membrane systems evolved from specialized invaginations of the prokaryotic plasma membrane, it is not clear how the nuclear pore complex could have evolved from organisms with no analogous transport system. Here we use computational and biochemical methods to perform a structural analysis of the seven proteins comprising the yNup84/vNup107-160 subcomplex, a core building block of the nuclear pore complex. Our analysis indicates that all seven proteins contain either a beta-propeller fold, an alpha-solenoid fold, or a distinctive arrangement of both, revealing close similarities between the structures comprising the yNup84/vNup107-160 subcomplex and those comprising the major types of vesicle coating complexes that maintain vesicular trafficking pathways. These similarities suggest a common evolutionary origin for nuclear pore complexes and coated vesicles in an early membrane-curving module that led to the formation of the internal membrane systems in modern eukaryotes.
Conflict of interest statement
The authors have declared that no conflicts of interest exist.
Figures
Figure 1. Ribbon Representation of Nup Models
β-sheets (β-propellers) are colored cyan and α-helices (α-solenoids) are colored magenta. Gray dashed lines indicate regions that were not modeled. Arrowheads indicate the positions of high proteolytic susceptibility (see Figures 2 and 3).
Figure 2. Proteolytic Domain Map of the Yeast Nup84 Subcomplex Proteins
Immunoblots of limited proteolysis digests for Protein A-tagged versions of each of the seven nups in the yNup84 subcomplex. Each protein is detected via its carboxyl-terminal tag; thus, all the fragments visualized are amino-terminal truncations (except for the full length proteins, which are indicated by arrowheads). The fragments of the Asp-N and Lys-C protease digests depicted in Figure 2 are labeled with letters (A, B, C…) that correspond to those in Table 2, and the terminal Protein A fragments are labeled with an X (the Protein A tag is resistant to proteolysis). The sizes of marker proteins are indicated in kilodaltons (kDa) to the right of the gel.
Figure 3. Predicted Secondary Structure Maps of the Nup84 Subcomplex Proteins
Thin horizontal lines represent the primary sequence of each protein; secondary structure predictions are shown as columns above each line for β-strands (β-propellers; cyan) and α-helices (α-solenoids; magenta). The height of the columns is proportional to the confidence of the secondary structure prediction (McGuffin et al. 2000). The modeled regions are indicated above each sequence by horizontal dark bars, corresponding to the models in Figure 1. Proteolytic cleavage sites are identified by small, medium, and large arrows for weak, medium, and strong susceptibility sites, respectively. Where necessary, uncertainties in the precise cleavage positions are indicated above the arrows by horizontal bars.
Figure 4. The Nup84 Complex and Coated Vesicles Share a Common Architecture
A diagram showing the organization of the clathrin/AP-2 coated vesicle complex is shown at left; the positions of clathrin and the adaptin AP-2 large subunits (α, β2 plus “ear” domains) and small subunits (σ, μ) are indicated. β-propeller regions are colored cyan, α-solenoid regions are colored magenta, and sample ribbon models for each fold are shown in the center. The variants of each fold that are found as domains in major components of the three kinds of vesicle-coating complexes and the yNup84 subcomplex are listed on the right. The -N and -C indicate amino-terminal and carboxyl-terminal domains, respectively. The classification of these domains is based on X-ray crystallography data (clathrin, α-adaptin, β2-adaptin [PDB codes 1gw5, 1bpo, 1b89 (ter Haar et al. 1998; Collins et al. 2002)]), by the detailed homology modeling presented here (yNup84 complex proteins; ySec13 also in Saxena et al. [1996]), or by sequence homology or unpublished secondary structure prediction and preliminary analyses (COPI I (sec31) complex proteins [Schledzewski et al. 1999], Sec31).
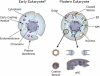
Figure 5. Proposed Model for the Evolution of Coated Vesicles and Nuclear Pore Complexes
Early eukaryotes (left) acquired a membrane-curving protein module (purple) that allowed them to mold their plasma membrane into internal compartments and structures. Modern eukaryotes have diversified this membrane-curving module into many specialized functions (right), such as endocytosis (orange), ER and Golgi transport (green and brown), and NPC formation (blue). This module (pink) has been retained in both NPCs (right bottom) and coated vesicles (left bottom), as it is needed to stabilize curved membranes in both cases.
Similar articles
- Structural evolution of the membrane-coating module of the nuclear pore complex.
Liu X, Mitchell JM, Wozniak RW, Blobel G, Fan J. Liu X, et al. Proc Natl Acad Sci U S A. 2012 Oct 9;109(41):16498-503. doi: 10.1073/pnas.1214557109. Epub 2012 Sep 26. Proc Natl Acad Sci U S A. 2012. PMID: 23019579 Free PMC article. - Simple fold composition and modular architecture of the nuclear pore complex.
Devos D, Dokudovskaya S, Williams R, Alber F, Eswar N, Chait BT, Rout MP, Sali A. Devos D, et al. Proc Natl Acad Sci U S A. 2006 Feb 14;103(7):2172-7. doi: 10.1073/pnas.0506345103. Epub 2006 Feb 6. Proc Natl Acad Sci U S A. 2006. PMID: 16461911 Free PMC article. - Nuclear pore complex-a coat specifically tailored for the nuclear envelope.
Onischenko E, Weis K. Onischenko E, et al. Curr Opin Cell Biol. 2011 Jun;23(3):293-301. doi: 10.1016/j.ceb.2011.01.002. Epub 2011 Feb 4. Curr Opin Cell Biol. 2011. PMID: 21296566 Free PMC article. Review. - Comparative genomics, evolution and origins of the nuclear envelope and nuclear pore complex.
Mans BJ, Anantharaman V, Aravind L, Koonin EV. Mans BJ, et al. Cell Cycle. 2004 Dec;3(12):1612-37. doi: 10.4161/cc.3.12.1316. Epub 2004 Dec 20. Cell Cycle. 2004. PMID: 15611647 - Nuclear pore complexes: round the bend?
Antonin W, Mattaj IW. Antonin W, et al. Nat Cell Biol. 2005 Jan;7(1):10-2. doi: 10.1038/ncb0105-10. Nat Cell Biol. 2005. PMID: 15632943 Review. No abstract available.
Cited by
- SEA you later alli-GATOR--a dynamic regulator of the TORC1 stress response pathway.
Dokudovskaya S, Rout MP. Dokudovskaya S, et al. J Cell Sci. 2015 Jun 15;128(12):2219-28. doi: 10.1242/jcs.168922. Epub 2015 May 1. J Cell Sci. 2015. PMID: 25934700 Free PMC article. Review. - Molecular architecture of the inner ring scaffold of the human nuclear pore complex.
Kosinski J, Mosalaganti S, von Appen A, Teimer R, DiGuilio AL, Wan W, Bui KH, Hagen WJ, Briggs JA, Glavy JS, Hurt E, Beck M. Kosinski J, et al. Science. 2016 Apr 15;352(6283):363-5. doi: 10.1126/science.aaf0643. Science. 2016. PMID: 27081072 Free PMC article. - Architecture of the symmetric core of the nuclear pore.
Lin DH, Stuwe T, Schilbach S, Rundlet EJ, Perriches T, Mobbs G, Fan Y, Thierbach K, Huber FM, Collins LN, Davenport AM, Jeon YE, Hoelz A. Lin DH, et al. Science. 2016 Apr 15;352(6283):aaf1015. doi: 10.1126/science.aaf1015. Epub 2016 Apr 14. Science. 2016. PMID: 27081075 Free PMC article. - Intraflagellar transport trains and motors: Insights from structure.
Webb S, Mukhopadhyay AG, Roberts AJ. Webb S, et al. Semin Cell Dev Biol. 2020 Nov;107:82-90. doi: 10.1016/j.semcdb.2020.05.021. Epub 2020 Jul 16. Semin Cell Dev Biol. 2020. PMID: 32684327 Free PMC article. Review. - Nuclear transport is becoming crystal clear.
Madrid AS, Weis K. Madrid AS, et al. Chromosoma. 2006 Apr;115(2):98-109. doi: 10.1007/s00412-005-0043-3. Epub 2006 Jan 19. Chromosoma. 2006. PMID: 16421734 Review. No abstract available.
References
- Amos LA, van den Ent F, Lowe J. Structural/functional homology between the bacterial and eukaryotic cytoskeletons. Curr Opin Cell Biol. 2004;16:24–31. - PubMed
- Andrade MA, Perez-Iratxeta C, Ponting CP. Protein repeats: Structures, functions, and evolution. J Struct Biol. 2001a;134:117–131. - PubMed
- Andrade MA, Petosa C, O'Donoghue SI, Muller CW, Bork P. Comparison of ARM and HEAT protein repeats. J Mol Biol. 2001b;309:1–18. - PubMed
- Bednenko J, Cingolani G, Gerace L. Nucleocytoplasmic transport: Navigating the channel. Traffic. 2003;4:127–135. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R33 CA089810/CA/NCI NIH HHS/United States
- P41 RR000862/RR/NCRR NIH HHS/United States
- R33 CA89810/CA/NCI NIH HHS/United States
- GM062427/GM/NIGMS NIH HHS/United States
- RR00862/RR/NCRR NIH HHS/United States
- R01 GM071329/GM/NIGMS NIH HHS/United States
- R01 GM062427/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases