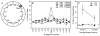Hippocampus and remote spatial memory in rats - PubMed (original) (raw)
Hippocampus and remote spatial memory in rats
Robert E Clark et al. Hippocampus. 2005.
Abstract
Damage to the hippocampus typically produces temporally graded retrograde amnesia, whereby memories acquired recently are impaired more than memories acquired remotely. This phenomenon has been demonstrated repeatedly in a variety of species and tasks. It has also figured prominently in theoretical treatments of memory and hippocampal function. Yet temporally graded retrograde amnesia has not been demonstrated following hippocampal damage in spatial tasks like the water maze. We have assessed recent and remote spatial memory following hippocampal lesions in three different tests of spatial memory: (1) the standard water maze; (2) the Oasis maze, a dry-land version of the water maze; and (3) the annular water maze, where training and testing occur within a circular corridor. Training protocols were developed for each task such that retention of spatial memory could be expressed after very long retention intervals. In addition, retention in each task was assessed with single probe trials so that the assessment of remote memory did not depend on the ability to relearn across multiple trials. The findings were consistent across the three tasks. In the standard water maze (Experiment 1), spatial memory was impaired after training-surgery intervals of 1 day, 8 weeks, or 14 weeks. Similarly, in the Oasis maze (Experiment 2), spatial memory was impaired after training-surgery intervals of 1 day and 9 weeks. Finally, in the annular water maze (Experiment 3), spatial memory was impaired after training-surgery intervals of 9 weeks and 14 weeks. Dorsal hippocampal lesions impaired performance to the same extent as complete lesions. The impairment in remote spatial memory could reflect disruption of previously acquired spatial information. Alternatively, it is possible that in these tasks hippocampal lesions might produce an impairment in performance that prevents the expression of an otherwise intact spatial memory.
(c) 2004 Wiley-Liss, Inc.
Figures
FIGURE 1
Top view of the three spatial tasks. All three tasks require the use of distal spatial cues (e.g., posters located on testing room walls). A: Water maze (Experiment 1). Rats were trained to find an escape platform hidden just below the surface of the water. B: Oasis maze (Experiment 2). Water-deprived rats were trained to find a single well containing a 0.3 ml drop of water among 426 wells. C: Annular water maze (Experiment 3). Rats were trained to find an escape platform hidden just below the surface of the water. The swim path was restricted by clear walls that formed an annulus, such that rats necessarily encountered the platform location in the course of swimming. Recognition of the platform location during probe trials was indicated by a slowing of swim speed in the vicinity of the platform. Scale bar = 1 m for all three mazes.
FIGURE 2
Reconstructions of coronal sections showing the largest (striped) and smallest (black) area of damage for rats with large hippocampal lesions (H-RF) and dorsal hippocampal lesions (DH-RF). Reconstructions from rats in Experiment 1(A), Experiment 2 (B), and Experiment 3 (C). Each series of sections progresses (top to bottom) from anterior to posterior levels. Numbers represent the distance in millimeters posterior to bregma.
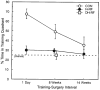
FIGURE 3
Water maze performance (Experiment 1). Percentage time in the training quadrant during a retention probe given 14 days after surgery. Control rats (CON), rats with complete hippocampal lesions (H-RF), and rats with dorsal hippocampal lesions (DH-RF) were tested for retention after a 1-day (CON, n = 8; H-RF, n = 8), 8-week (CON, n = 16; H-RF, n = 9; DH-RF, n = 8), or 14-week (CON, n = 12; H-RF, n = 12) training–surgery interval. None of the lesion groups performed above chance (25%). The 1-day and 8-week control groups performed above chance and better than the corresponding lesion group. Error bars = SEM.
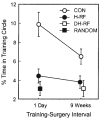
FIGURE 4
Performance on the Oasis maze (Experiment 2). Percentage of time in the training circle during a retention probe trial that was given 14 days after surgery. Control rats (CON) and rats with complete hippocampal lesions (H-RF) or dorsal hippocampal lesions (DH-RF) were tested for retention after a 1-day (CON, n = 16; H-RF, n = 16) or 9-week (CON, n = 24; H-RF, n = 22; DH-RF, n = 8) training–surgery interval. None of the lesion groups performed better than rats that were presented on each trial with random water locations (RANDOM, n = 12). Mean performance of the RANDOM group is represented by the filled square. The 1-day and 9-week control groups performed better than the corresponding lesion groups and better than the RANDOM group. Error bars = SEM.
FIGURE 5
Performance on the annular water maze (Experiment 3). A: For scoring, twelve 30° arc zones were constructed around the training (platform location) zone. Chance performance is 8.3% (100% divided by 12 zones). B: Percentage time spent by each group in the 12 zones during the retention probe trial that was given 14 days after surgery. Memory of the platform location is indicated as increased percentage time in the training zone. C: Percentage time in the training zone replotted as a function of training–surgery interval. For rats with complete hippocampal lesions (H-RF), surgery occurred 9 weeks or 14 weeks after training (n = 7 for all groups). None of the lesion groups performed above chance (8.3%). Both the 9-week CON and the 14-week CON groups performed better than the corresponding lesion groups. Error bars = SEM.
Similar articles
- Impaired remote spatial memory after hippocampal lesions despite extensive training beginning early in life.
Clark RE, Broadbent NJ, Squire LR. Clark RE, et al. Hippocampus. 2005;15(3):340-6. doi: 10.1002/hipo.20076. Hippocampus. 2005. PMID: 15744736 Free PMC article. - Retrograde amnesia: neither partial nor complete hippocampal lesions in rats result in preferential sparing of remote spatial memory, even after reminding.
Martin SJ, de Hoz L, Morris RG. Martin SJ, et al. Neuropsychologia. 2005;43(4):609-24. doi: 10.1016/j.neuropsychologia.2004.07.007. Neuropsychologia. 2005. PMID: 15716151 - Hippocampal activation during the recall of remote spatial memories in radial maze tasks.
Schlesiger MI, Cressey JC, Boublil B, Koenig J, Melvin NR, Leutgeb JK, Leutgeb S. Schlesiger MI, et al. Neurobiol Learn Mem. 2013 Nov;106:324-33. doi: 10.1016/j.nlm.2013.05.007. Epub 2013 Jun 4. Neurobiol Learn Mem. 2013. PMID: 23742919 - Retrograde amnesia after hippocampal damage: recent vs. remote memories in two tasks.
Sutherland RJ, Weisend MP, Mumby D, Astur RS, Hanlon FM, Koerner A, Thomas MJ, Wu Y, Moses SN, Cole C, Hamilton DA, Hoesing JM. Sutherland RJ, et al. Hippocampus. 2001;11(1):27-42. doi: 10.1002/1098-1063(2001)11:1<27::AID-HIPO1017>3.0.CO;2-4. Hippocampus. 2001. PMID: 11261770 Review. - The cognitive neuroscience of remote episodic, semantic and spatial memory.
Moscovitch M, Nadel L, Winocur G, Gilboa A, Rosenbaum RS. Moscovitch M, et al. Curr Opin Neurobiol. 2006 Apr;16(2):179-90. doi: 10.1016/j.conb.2006.03.013. Epub 2006 Mar 27. Curr Opin Neurobiol. 2006. PMID: 16564688 Review.
Cited by
- Western diet consumption impairs memory function via dysregulated hippocampus acetylcholine signaling.
Hayes AMR, Lauer LT, Kao AE, Sun S, Klug ME, Tsan L, Rea JJ, Subramanian KS, Gu C, Tanios N, Ahuja A, Donohue KN, Décarie-Spain L, Fodor AA, Kanoski SE. Hayes AMR, et al. Brain Behav Immun. 2024 May;118:408-422. doi: 10.1016/j.bbi.2024.03.015. Epub 2024 Mar 8. Brain Behav Immun. 2024. PMID: 38461956 Free PMC article. - Effect of lidocaine on cognitively impaired rats: Anti-inflammatory and antioxidant mechanisms in combination with CRMP2 antiphosphorylation.
Zheng X, Lin Y, Huang L, Lin X. Zheng X, et al. Immun Inflamm Dis. 2023 Oct;11(10):e1040. doi: 10.1002/iid3.1040. Immun Inflamm Dis. 2023. PMID: 37904712 Free PMC article. - Western diet consumption impairs memory function via dysregulated hippocampus acetylcholine signaling.
Hayes AMR, Lauer LT, Kao AE, Sun S, Klug ME, Tsan L, Rea JJ, Subramanian KS, Gu C, Tanios N, Ahuja A, Donohue KN, Décarie-Spain L, Fodor AA, Kanoski SE. Hayes AMR, et al. bioRxiv [Preprint]. 2023 Jul 25:2023.07.21.550120. doi: 10.1101/2023.07.21.550120. bioRxiv. 2023. PMID: 37546790 Free PMC article. Updated. Preprint. - Improvement of Spatial Memory and Cognitive Function in Mice via the Intervention of Milk Fat Globule Membrane.
Zhou Y, Zou X, Feng R, Zhan X, Hong H, Luo Y, Tan Y. Zhou Y, et al. Nutrients. 2023 Jan 19;15(3):534. doi: 10.3390/nu15030534. Nutrients. 2023. PMID: 36771241 Free PMC article. - Evolution of memory system-related genes.
Bajaffer A, Mineta K, Gojobori T. Bajaffer A, et al. FEBS Open Bio. 2021 Dec;11(12):3201-3210. doi: 10.1002/2211-5463.13224. Epub 2021 Jun 28. FEBS Open Bio. 2021. PMID: 34110105 Free PMC article. Review.
References
- Amaral DG, Witter MP. Hippocampal formation. In: Paxinos G, editor. The rat nervous system. 2nd ed. Academic Press; San Diego, CA: 1995. pp. 443–493.
- Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus. 2001;11:8–17. - PubMed
- Bolhuis J, Stewart CA, Forrest EM. Retrograde amnesia and memory reactivation in rats with ibotenate lesions to the hippocampus or subiculum. Q J Exp Psychol B. 1994;47:129–150. - PubMed
- Bontempi B, Laurent-Demir C, Destrade C, Jaffard R. Time-dependent reorganization of brain circuitry underlying long-term memory storage. Nature. 1999;400:671–675. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical