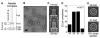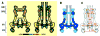Structural insights into the assembly of the type III secretion needle complex - PubMed (original) (raw)
Structural insights into the assembly of the type III secretion needle complex
Thomas C Marlovits et al. Science. 2004.
Abstract
Type III secretion systems (TTSSs) mediate translocation of virulence factors into host cells. We report the 17-angstrom resolution structures of a central component of Salmonella typhimurium TTSS, the needle complex, and its assembly precursor, the bacterial envelope-anchored base. Both the base and the fully assembled needle complex adopted multiple oligomeric states in vivo, and needle assembly was accompanied by recruitment of the protein PrgJ as a structural component of the base. Moreover, conformational changes during needle assembly created scaffolds for anchoring both PrgJ and the needle substructure and may provide the basis for substrate-specificity switching during type III secretion.
Figures
Fig. 1
The needle complex and the base complex of the TTSS from S. typhimurium can adopt different symmetries in vivo. (A) Nomenclature of the structural features of the needle complex. The needle complex is divided into two distinctive substructures: the membrane-embedded base and the extracellular needle filament. The base spans the periplasm and is associated with the inner and outer membranes, where ringlike structures are visible in electron micrographs of negatively stained needle complexes (2% phosphotungstic acid, pH 7) (B). The outer membrane–associated rings (OR1 and OR2) are composed of the protein InvG, and the inner membrane–associated rings (IR1 and IR2) contain the proteins PrgH and PrgK (). The only protein identified for the needle filament to date is PrgI (). Bar, 30 nm. (C) Model-based multireference alignment revealed significant differences in the diameters of the average projections obtained for different rotational symmetries, as indicated by white arrows in the comparison of the IR1 of the 19- and 22-fold particles. (D) Distribution of different symmetries in needle complexes isolated from wild-type S. typhimurium. The data were generated by examining 3577 particles. (E) After sorting of the particles and 3D reconstruction without enforcing any symmetry, the true rotational symmetries could be derived from cross sections through IR1 of the reconstructed needle complexes, as shown for the 20- and 21-fold particles.
Fig. 2
Surface renderings of the structures of the base (A) and the needle complex (B) show individual subunits within the IR rings, but the OR rings appear smooth. The structure shown here is that of the 20-fold complex with 20-fold symmetry imposed. The absolute hand of the reconstruction was not determined. (A) The contouring threshold represents ~120% of the expected mass of the 20-fold base (2.69 MDa), assuming a protein density of 0.844 dalton/Å3 (IMAGIC-5, Image Science Software GmbH, Germany) and including a 13% contribution of the detergent bound to the two membrane-embedded regions of the complex. Owing to the uncertainty in the number of PrgI and PrgJ subunits present in the final reconstruction of the needle complex (B), the clearly defined subunits of IR1 of the base were used as a point of reference for thresholding of the needle complex because this part of IR1 is largely unaffected by the conformational changes during needle assembly and, hence, should closely match the appearance in the base (movie S2). Structural differences between the base and needle complex are described in the text. (C) Removal of the front half of the base shows its internal chamber. A socketlike structure, marked by an asterisk, extends into the chamber’s interior and serves as an anchoring point for the “inner rod” in the needle complex.
Fig. 3
Needle assembly induces large conformational changes in the base. (A) Contoured longitudinal sections show the distribution of protein density within the base and needle complexes. Protein densities are represented by 14 evenly spaced contour levels starting at 1σ above the mean densities of the volumes. (B) An overlay of the longitudinal sections for the base (blue) and needle complex (gray) reveals regions of high conformational flexibility (light blue). (C) Key dimensions of the needle complex, given in Å, were measured from center to center because such measurements are independent of contouring thresholds and increments.
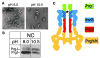
Fig. 4
PrgJ is recruited as a structural component to the base during needle assembly. (A) Electron micrographs of needle complexes before (pH 8.0) and after subjection to pH 10.5 to affect needle disassembly. (B) Western blot analysis of untreated and pH 10.5–treated needle showed that elevated pH drastically diminished the amount of the needle protein PrgI, as expected based on the images shown in (A), but did not affect the amount of PrgJ, which, therefore, must be located within the base. (C) Model cartoon summarizing the proposed organization of the five major structural components of the needle complex: PrgH, PrgK, PrgJ, InvG, and PrgI. Hatched coloring indicates the uncertainty in the exact boundaries of PrgI, InvG, and PrgJ. The asterisk marks the location where the secretion tunnel markedly narrows at the entry point to the needle, which attaches to the outermost periphery of the base through a contact with InvG.
_## Similar articles
* Topology and organization of the Salmonella typhimurium type III secretion needle complex components. Schraidt O, Lefebre MD, Brunner MJ, Schmied WH, Schmidt A, Radics J, Mechtler K, Galán JE, Marlovits TC. Schraidt O, et al. PLoS Pathog. 2010 Apr 1;6(4):e1000824. doi: 10.1371/journal.ppat.1000824. PLoS Pathog. 2010. PMID: 20368966 Free PMC article. * Three-dimensional model of Salmonella's needle complex at subnanometer resolution. Schraidt O, Marlovits TC. Schraidt O, et al. Science. 2011 Mar 4;331(6021):1192-5. doi: 10.1126/science.1199358. Science. 2011. PMID: 21385715 * Supramolecular structure of the Salmonella typhimurium type III protein secretion system. Kubori T, Matsushima Y, Nakamura D, Uralil J, Lara-Tejero M, Sukhan A, Galán JE, Aizawa SI. Kubori T, et al. Science. 1998 Apr 24;280(5363):602-5. doi: 10.1126/science.280.5363.602. Science. 1998. PMID: 9554854 * Structures of Type III Secretion System Needle Filaments. Habenstein B, El Mammeri N, Tolchard J, Lamon G, Tawani A, Berbon M, Loquet A. Habenstein B, et al. Curr Top Microbiol Immunol. 2020;427:109-131. doi: 10.1007/82_2019_192. Curr Top Microbiol Immunol. 2020. PMID: 31974760 Review. * Imaging the assembly, structure and activity of type III secretion systems. Enninga J, Rosenshine I. Enninga J, et al. Cell Microbiol. 2009 Oct;11(10):1462-70. doi: 10.1111/j.1462-5822.2009.01360.x. Epub 2009 Jul 20. Cell Microbiol. 2009. PMID: 19622097 Review.
## Cited by
* The blueprint of the type-3 injectisome. Kosarewicz A, Königsmaier L, Marlovits TC. Kosarewicz A, et al. Philos Trans R Soc Lond B Biol Sci. 2012 Apr 19;367(1592):1140-54. doi: 10.1098/rstb.2011.0205. Philos Trans R Soc Lond B Biol Sci. 2012. PMID: 22411984 Free PMC article. Review. * Secretion systems in Gram-negative bacteria: structural and mechanistic insights. Costa TR, Felisberto-Rodrigues C, Meir A, Prevost MS, Redzej A, Trokter M, Waksman G. Costa TR, et al. Nat Rev Microbiol. 2015 Jun;13(6):343-59. doi: 10.1038/nrmicro3456. Nat Rev Microbiol. 2015. PMID: 25978706 Review. * The cytoplasmic domain of MxiG interacts with MxiK and directs assembly of the sorting platform in the Shigella type III secretion system. Tachiyama S, Chang Y, Muthuramalingam M, Hu B, Barta ML, Picking WL, Liu J, Picking WD. Tachiyama S, et al. J Biol Chem. 2019 Dec 13;294(50):19184-19196. doi: 10.1074/jbc.RA119.009125. Epub 2019 Nov 7. J Biol Chem. 2019. PMID: 31699894 Free PMC article. * Secretion signal recognition by YscN, the Yersinia type III secretion ATPase. Sorg JA, Blaylock B, Schneewind O. Sorg JA, et al. Proc Natl Acad Sci U S A. 2006 Oct 31;103(44):16490-5. doi: 10.1073/pnas.0605974103. Epub 2006 Oct 18. Proc Natl Acad Sci U S A. 2006. PMID: 17050689 Free PMC article. * High-resolution view of the type III secretion export apparatus in situ reveals membrane remodeling and a secretion pathway. Butan C, Lara-Tejero M, Li W, Liu J, Galán JE. Butan C, et al. Proc Natl Acad Sci U S A. 2019 Dec 3;116(49):24786-24795. doi: 10.1073/pnas.1916331116. Epub 2019 Nov 19. Proc Natl Acad Sci U S A. 2019. PMID: 31744874 Free PMC article.
## References
1. 1. Cornelis GR, Van Gijsegem F. Annu Rev Microbiol. 2000;54:735. - PubMed 2. 1. Galán JE, Collmer A. Science. 1999;284:1322. - PubMed 3. 1. Kubori T, et al. Science. 1998;280:602. - PubMed 4. 1. Kubori T, Sukhan A, Aizawa SI, Galán JE. Proc Natl Acad Sci USA. 2000;97:10225. - PMC - PubMed 5. 1. Galán JE, Curtiss R., III Proc Natl Acad Sci USA. 1989;86:6383. - PMC - PubMed
## Publication types
## MeSH terms
## Substances
## Grants and funding
* R01 GM066145/GM/NIGMS NIH HHS/United States * GM35433/GM/NIGMS NIH HHS/United States * P42 RR-01081/RR/NCRR NIH HHS/United States * AI30492/AI/NIAID NIH HHS/United States * GM66145/GM/NIGMS NIH HHS/United States
## LinkOut - more resources
* ### Full Text Sources * Atypon * Europe PubMed Central * Nature Publishing Group * Ovid Technologies, Inc. * PubMed Central * ### Other Literature Sources * H1 Connect - Access expert opinions and insights on biomedical research. * The Lens - Patent Citations Database