Number of T reg cells that differentiate does not increase upon encounter of agonist ligand on thymic epithelial cells - PubMed (original) (raw)
Comparative Study
. 2004 Nov 15;200(10):1221-30.
doi: 10.1084/jem.20041022. Epub 2004 Nov 8.
Affiliations
- PMID: 15534371
- PMCID: PMC2211925
- DOI: 10.1084/jem.20041022
Comparative Study
Number of T reg cells that differentiate does not increase upon encounter of agonist ligand on thymic epithelial cells
Hisse-Martien van Santen et al. J Exp Med. 2004.
Abstract
It has been reported that the differentiation of CD4+CD25+ regulatory T cells (T reg cells) can be induced by agonist peptide/major histocompatibility complex ligands in the thymus. Exploiting a transgenic mouse line wherein expression of a particular T cell epitope can be controlled temporally and quantitatively, we found that diversion of differentiating thymocytes into the FoxP3 T reg cell pathway by this agonist ligand was essentially nonexistent. However, CD4+CD25+ thymocytes were much less sensitive than their CD4+CD25- companions, by two to three orders of magnitude, to agonist-induced clonal deletion, such that their proportion increased, giving the false impression of induced differentiation. To account for these and prior observations, one can propose that differentiation along the CD4+CD25+ pathway is induced by cues other than recognition of self-agonist cues, which are poorly read by thymocytes, whose T cell receptors are conducive to selection toward the conventional CD4+CD25- lineage. Thus, selective survival, rather than induced differentiation, may explain the apparent enrichment observed here and in previous studies.
Figures
Figure 1.
Tet-controlled expression of a T cell epitope. (A) Schematic depiction of the tet-responsive double transgenic system. A transactivator (TA) consisting of the _tet_-repressor (TetR) fused to the VP16-activating domain is expressed via the Eα promoter. Binding of the transactivator to tet operator (TetO) regulatory sequences upstream of the minimal CMV promoter is necessary to drive expression of a modified invariant chain protein and can be blocked by tet. The modified invariant chain construct (TIM) contains a MCC–derived, I-Ek–restricted minimal T cell epitope instead of the CLIP region. (B) Predominant expression of the TIM transgene in the thymus. RNA isolated from the indicated organs was analyzed via S1 nuclease protection assays for transcription of the TIM gene. The DNA probe used in this assay was complementary to the first 174 bases of the recombinant invariant chain cDNA and 54 bases from the upstream rabbit β-globin exon sequence, allowing discrimination of TIM transcripts from the endogenous invariant chain (Ii) transcripts on the basis of a difference in size of the protected fragments. (C) Immunohistochemical analysis of the thymus from invariant chain-deficient TA × TIM mice. Adjacent frozen tissue sections were stained with the anti–MHC class II mAb M5/114 and the antimurine invariant chain mAb IN1.1. Note that, due to the absence of endogenous invariant chain, the IN1.1 antibody reveals expression of the TIM transgene. (D) Differentiation of AND thymocytes in BM chimeras. Profiles show the CD8 versus CD4 distribution and expression of the clonotypic Vα11 and Vβ3 TCR chains on mature CD4+ T cells in the indicated chimeras. Note that little, if any, negative selection of AND thymocytes occurs when only BM-derived cells are positive for the TA and TIM genes.
Figure 2.
Increased proportion of CD4+CD25+ T reg cells upon increase in TIM expression. (A) The amount of TIM transcripts can be regulated by graded doses of tet. TA × TIM animals were treated for life with the indicated doses of tet. Thymic RNA was analyzed for TIM RNA transcripts via real-time PCR (TaqMan). Mice in the control group lacked either the transactivator or reporter transgene. n.d., not detected. (B) Thymus (top) and LNs (bottom) from TAND mice treated for life with tet were analyzed at 7 wk of age by flow cytometry for differentiation of AND-transgenic CD4+CD25+ T reg cells. Clonotype positive T cells were identified by staining with mAbs against the Vα11 and Vβ3 TCR chains. Animals shown in this panel were treated with, from left to right, 100, 60, 20, and 10 mg/l tet in the drinking water.
Figure 3.
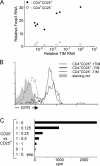
Characterization of CD4+CD25+ thymocytes in TAND mice. (A) RNA extracted from one thymic lobe of TAND animals treated with graded doses of tet was analyzed by real-time PCR for the relative amount of TIM RNA. CD4+CD25+ and CD4+CD25− thymocytes were sorted from the other lobe, and RNA purified from these cell populations was assayed by real-time PCR for the relative expression level of Foxp3 transcripts. Relative Foxp3 RNA levels were plotted as a function of TIM RNA transcripts levels. (B) CD4+CD25+ thymocytes in TAND mice express GITR. Vβ3+CD4+CD25+ thymocytes from animals treated for life with 250 mg/l (black line, no TIM message detected) or 40 mg/l (gray line) tet and Vβ3+CD4+CD25− thymocytes from animals treated with 250 mg/l tet (dotted black line) were analyzed for cell surface expression of GITR via flow cytometry. The solid gray histogram shows staining with affinity-purified control antibodies. (C) CD4+CD25+ thymocytes from a TAND mouse treated with 50 mg/l tet were purified via cell sorting using mAbs against CD4, CD8, and CD25. Staining with mAbs against both TCR chains was omitted to avoid potential stimulatory or inhibitory effects on the T reg cells. Increasing numbers of these cells were added to wells containing fixed numbers of CD4+CD25− AND T cells. Cultures were grown for 3 d in the presence of 10 μM MCC88–103 peptide, and [3H]thymidine was added during the last 16 h of culture.
Figure 4.
Enumeration of thymocyte populations in tet-treated TAND mice. (A) The proportion of clonotype positive CD4+CD25+ thymocytes amongst CD4+ thymocytes is dependent on the expression level of the TIM transgene. (B) Absolute number of clonotype positive CD4+ CD25− (open circles) and CD4+CD25+ (closed diamonds) thymocytes in tet-treated TAND mice, based on the analysis shown in Fig. 2, as a function of the relative expression level of TIM RNA in the thymus of these animals. Control animals lacked either the transactivator or reporter transgene. (C) Absolute numbers (as million cells/whole thymus) of clonotype positive (closed diamonds) and clonotype negative (open triangles) CD4+CD25+ thymocytes in thymi of tet-treated TAND mice. The relative increase in clonotype positive cells upon encounter with increasing amounts of the agonist ligand is very modest. Note that, at the highest doses attainable in this system, clonotype positive CD4+CD25+ thymocytes undergo clonal deletion. (D) Representative examples showing the percentage of clonotype positive CD4+CD25+ thymocytes are shown for a range of TIM doses (indicated by black arrows).
Figure 5.
Factors determining the relative size of the CD4+CD25+ compartment. This model, which attempts to group information from previous studies and the present work, proposes that two main factors that determine the efficiency via which thymocyte precursors (gray circles) are directed toward the conventional CD4+CD25− pathway (blue circles) or the CD4+CD25+ T reg cell pathway (red circles). First, the poor ability of MHC II–restricted thymocytes to become T reg cells as noted quasi-universally in MHC II–restricted TCR-transgenic (Tg) systems. This is probably not unexpected, as there is an “experimental bias” in the Tg lines analyzed by the community, with a strong selection for lines that show robust MHC II selection to the conventional CD4+ compartment. Second, the relative resistance of CD4+CD25+ T reg cell thymocytes to clonal deletion induced by MHC II/p ligands is clearly established in this paper. The model proposes that the relative changes between populations can be accounted for by a competitive balance between these two forces: when T precursors express a TCR that promotes very efficient selection to the conventional CD4SP pathway, the CD4+CD25+ T reg cell lineage is underrepresented, and this is particularly true when the RAG deficiency prevents the rescue of the CD4+CD25+ T reg cell pathway through endogenous TCRs. In contrast, the presence of agonist ligand, which selectively eliminates conventional CD4+CD25− cells, brings forth the T reg cell pathway.
Similar articles
- Peptide specificity of thymic selection of CD4+CD25+ T cells.
Pacholczyk R, Kraj P, Ignatowicz L. Pacholczyk R, et al. J Immunol. 2002 Jan 15;168(2):613-20. doi: 10.4049/jimmunol.168.2.613. J Immunol. 2002. PMID: 11777953 - A role for accessibility to self-peptide-self-MHC complexes in intrathymic negative selection.
Viret C, Sant'Angelo DB, He X, Ramaswamy H, Janeway CA Jr. Viret C, et al. J Immunol. 2001 Apr 1;166(7):4429-37. doi: 10.4049/jimmunol.166.7.4429. J Immunol. 2001. PMID: 11254698 - Autoantigen-independent deletion of diabetogenic CD4+ thymocytes by protective MHC class II molecules.
Schmidt D, Amrani A, Verdaguer J, Bou S, Santamaria P. Schmidt D, et al. J Immunol. 1999 Apr 15;162(8):4627-36. J Immunol. 1999. PMID: 10202002 - Thymic microenvironments for T-cell repertoire formation.
Nitta T, Murata S, Ueno T, Tanaka K, Takahama Y. Nitta T, et al. Adv Immunol. 2008;99:59-94. doi: 10.1016/S0065-2776(08)00603-2. Adv Immunol. 2008. PMID: 19117532 Review. - The role of self-peptides in the development of CD4+ CD25+ regulatory T cells.
Picca CC, Caton AJ. Picca CC, et al. Curr Opin Immunol. 2005 Apr;17(2):131-6. doi: 10.1016/j.coi.2005.01.003. Curr Opin Immunol. 2005. PMID: 15766671 Review.
Cited by
- Cellular and molecular determinants for the development of natural and induced regulatory T cells.
Yuan X, Malek TR. Yuan X, et al. Hum Immunol. 2012 Aug;73(8):773-82. doi: 10.1016/j.humimm.2012.05.010. Epub 2012 Jun 1. Hum Immunol. 2012. PMID: 22659217 Free PMC article. Review. - TCR ligand density and affinity determine peripheral induction of Foxp3 in vivo.
Gottschalk RA, Corse E, Allison JP. Gottschalk RA, et al. J Exp Med. 2010 Aug 2;207(8):1701-11. doi: 10.1084/jem.20091999. Epub 2010 Jul 26. J Exp Med. 2010. PMID: 20660617 Free PMC article. - Cathepsin L is essential for onset of autoimmune diabetes in NOD mice.
Maehr R, Mintern JD, Herman AE, Lennon-Duménil AM, Mathis D, Benoist C, Ploegh HL. Maehr R, et al. J Clin Invest. 2005 Oct;115(10):2934-43. doi: 10.1172/JCI25485. Epub 2005 Sep 22. J Clin Invest. 2005. PMID: 16184198 Free PMC article. - Plasticity of CD4(+) FoxP3(+) T cells.
Zhou X, Bailey-Bucktrout S, Jeker LT, Bluestone JA. Zhou X, et al. Curr Opin Immunol. 2009 Jun;21(3):281-5. doi: 10.1016/j.coi.2009.05.007. Epub 2009 Jun 6. Curr Opin Immunol. 2009. PMID: 19500966 Free PMC article. Review. - Retrovirus-specificity of regulatory T cells is neither present nor required in preventing retrovirus-induced bone marrow immune pathology.
Antunes I, Tolaini M, Kissenpfennig A, Iwashiro M, Kuribayashi K, Malissen B, Hasenkrug K, Kassiotis G. Antunes I, et al. Immunity. 2008 Nov 14;29(5):782-94. doi: 10.1016/j.immuni.2008.09.016. Immunity. 2008. PMID: 19006695 Free PMC article.
References
- Kappler, J.W., N. Roehm, and P. Marrack. 1987. T cell tolerance by clonal elimination in the thymus. Cell. 49:273–280. - PubMed
- Kisielow, P., H. Bluthmann, U.D. Staerz, M. Steinmetz, and H. von Boehmer. 1988. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 333:742–746. - PubMed
- Anderson, M.S., E.S. Venanzi, L. Klein, Z. Chen, S. Berzins, S.J. Turley, H. von Boehmer, R. Bronson, A. Dierich, C. Benoist, and D. Mathis. 2002. Projection of an immunological self-shadow within the thymus by the aire protein. Science. 298:139–1401. - PubMed
- Maloy, K.J., and F. Powrie. 2001. Regulatory T cells in the control of immune pathology. Nat. Immunol. 2:816–822. - PubMed
- Shevach, E.M. 2002. CD4+ CD25+ suppressor T cells: more questions than answers. Nat. Rev. Immunol. 2:389–400. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 DK036836/DK/NIDDK NIH HHS/United States
- R01 AI051530/AI/NIAID NIH HHS/United States
- 1 R01 AI51530-01/AI/NIAID NIH HHS/United States
- 2 P30 DK36836-17/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous