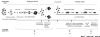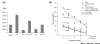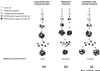Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells - PubMed (original) (raw)
Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells
Gabriela Dontu et al. Breast Cancer Res. 2004.
Abstract
Introduction: Notch signaling has been implicated in the regulation of cell-fate decisions such as self-renewal of adult stem cells and differentiation of progenitor cells along a particular lineage. Moreover, depending on the cellular and developmental context, the Notch pathway acts as a regulator of cell survival and cell proliferation. Abnormal expression of Notch receptors has been found in different types of epithelial metaplastic lesions and neoplastic lesions, suggesting that Notch may act as a proto-oncogene. The vertebrate Notch1 and Notch4 homologs are involved in normal development of the mammary gland, and mutated forms of these genes are associated with development of mouse mammary tumors.
Methods: In order to determine the role of Notch signaling in mammary cell-fate determination, we have utilized a newly described in vitro system in which mammary stem/progenitor cells can be cultured in suspension as nonadherent 'mammospheres'. Notch signaling was activated using exogenous ligands, or was inhibited using previously characterized Notch signaling antagonists.
Results: Utilizing this system, we demonstrate that Notch signaling can act on mammary stem cells to promote self-renewal and on early progenitor cells to promote their proliferation, as demonstrated by a 10-fold increase in secondary mammosphere formation upon addition of a Notch-activating DSL peptide. In addition to acting on stem cells, Notch signaling is also able to act on multipotent progenitor cells, facilitating myoepithelial lineage-specific commitment and proliferation. Stimulation of this pathway also promotes branching morphogenesis in three-dimensional Matrigel cultures. These effects are completely inhibited by a Notch4 blocking antibody or a gamma secretase inhibitor that blocks Notch processing. In contrast to the effects of Notch signaling on mammary stem/progenitor cells, modulation of this pathway has no discernable effect on fully committed, differentiated, mammary epithelial cells.
Conclusion: These studies suggest that Notch signaling plays a critical role in normal human mammary development by acting on both stem cells and progenitor cells, affecting self-renewal and lineage-specific differentiation. Based on these findings we propose that abnormal Notch signaling may contribute to mammary carcinogenesis by deregulating the self-renewal of normal mammary stem cells.
Figures
Figure 1
Effect of modulators of Notch signaling on self-renewal of mammary stem cells and on lineage commitment of mammary progenitor cells: experimental strategy. Treatment was applied (a) to mammospheres in suspension culture, (b) to mammosphere-derived cells cultivated on a collagen substratum, and (c) to differentiated cells cultivated on a collagen substratum. The assay for self-renewal was mammosphere formation. The assay for multilineage potential or lineage commitment was lineage-specific immunostaining of clonogenic cultures on the collagen substratum.
Figure 2
Effect of Notch agonist and antagonist treatment, assessed by Hes-1-driven luciferase expression in MCF-7 cells. (a) DSL treatment (100 nM) increased luciferase expression twofold compared with control, whereas treatment with the N4 blocking antibody (N4Ab) or gamma secretase inhibitor (GSI) (5 μM) reduced both endogenous and DSL-induced luciferase expression. (b) Dose–effect of Notch signaling antagonists. The difference in luciferase activity in cells treated with the maximum dose of inhibitory agent and in nontreated cells cells was statistically significant for each of the four different treatments (P < 0.005). The _R_2 values and trendlines for the four sets of experiments are shown.
Figure 3
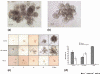
Effect of modulators of Notch signaling on self-renewal of mammary stem cells and on lineage commitment of mammary progenitor cells. (a)–(g) Effect of Notch agonist and antagonist treatment on primary and secondary mammosphere formation. Primary and secondary mammospheres were grown in standard conditions (a, d), in the presence of DSL peptide (b, e) and in the presence of N4 blocking antibody (N4Ab) (c, f). Primary mammospheres grown in the presence of N4 antibody (c) are smaller than the control (a). Secondary mammosphere formation is completely blocked by treatment with Notch4 blocking antibody (d, f). The addition of DSL peptide increases primary, secondary and tertiary mammosphere formation (a, b, d, e, g). Data are presented as the mean ± standard error of the mean. The calculated number of multilineage progenitor cells (number of spheres × % multilineage progenitors) shows a sevenfold increase after one passage, and a 100-fold increase after two passages, in mammospheres cultured in the presence of DSL compared with mammospheres cultured without added DSL. (h)–(k) Effect of Notch activation on lineage specification of human mammary progenitor cells. DSL treatment of mammospheres in suspension culture (DSL1) and on mammosphere-derived cells on the collagen substratum (DSL2) increases the number of myoepithelial progenitors, as shown by the clonogenic assay (h), and increases the rate of proliferation of bipotent and myoepithelial progenitors, as reflected by the colony size (i). DSL treatment in suspension culture increases the percentage of myoepithelial cells, as shown by flow-cytometry analysis (j). Cells were stained red (phycoerythrin [PE]) with myoepithelial marker CD10, and green (FITC) with the ductal epithelial marker epithelial-specific antigen (ESA). Treatment with DSL peptide does not have a lineage selective effect on differentiated cells, as shown by flow-cytometry analysis of human mammary epithelial cells passaged twice on collagen (k). Data are presented as the mean ± standard error of the mean.
Figure 4
Effect of Notch signaling on branching morphogenesis. (a) Fragment of the human mammary gland, after 8 hours of collagenase digestion of the reduction mammoplasty sample. (b) Complex ductal–alveolar structure developed in 18 days, from a mammosphere embedded in Matrigel. (c) Secondary mammospheres were embedded in Matrigel and treated with DSL peptide or Notch4 blocking antibody (N4Ab). Longer branches developed earlier in the presence of DSL peptide. (d) In addition, more spheres developed branching structures. The Notch4 blocking antibody completely inhibited proliferation and branching in Matrigel (c, d). Images shown in (c) are representative of 100–150 structures per experiment, scored in three different experiments with cells derived from three different patients. Data are presented as the mean ± standard error of the mean.
Figure 5
Notch4 protein detected by immunostaining (FITC-green) in structures derived from mammospheres embedded in Matrigel. (a),(b) Spherical structures at 5 days of cultivation (Nomarski contrast-phase image and immunofluorescence). (c),(d) Branching structures at 16 days of cultivation.
Figure 6
Effect of stem cell self-renewal and restrictive divisions on the efficiency of mammosphere formation. (a) Asymmetric stem cell self-renewal divisions result in constant mammosphere numbers in serial passages. (b) Proliferation of stem cells or progenitor cells through restrictive divisions results in a decreasing number of spheres in serial passages. (c) Symmetric self-renewal divisions of stem cells result in an increasing number of mammospheres in serial passages.
Similar articles
- Interactions between fibroblast growth factors and Notch regulate neuronal differentiation.
Faux CH, Turnley AM, Epa R, Cappai R, Bartlett PF. Faux CH, et al. J Neurosci. 2001 Aug 1;21(15):5587-96. doi: 10.1523/JNEUROSCI.21-15-05587.2001. J Neurosci. 2001. PMID: 11466430 Free PMC article. - Notch and epithelial-mesenchyme transition in development and tumor progression: another turn of the screw.
Grego-Bessa J, Díez J, Timmerman L, de la Pompa JL. Grego-Bessa J, et al. Cell Cycle. 2004 Jun;3(6):718-21. Epub 2004 Jun 28. Cell Cycle. 2004. PMID: 15197341 Review. - Notch signaling regulates mammary stem cell function and luminal cell-fate commitment.
Bouras T, Pal B, Vaillant F, Harburg G, Asselin-Labat ML, Oakes SR, Lindeman GJ, Visvader JE. Bouras T, et al. Cell Stem Cell. 2008 Oct 9;3(4):429-41. doi: 10.1016/j.stem.2008.08.001. Cell Stem Cell. 2008. PMID: 18940734 - Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells.
Liu S, Dontu G, Mantle ID, Patel S, Ahn NS, Jackson KW, Suri P, Wicha MS. Liu S, et al. Cancer Res. 2006 Jun 15;66(12):6063-71. doi: 10.1158/0008-5472.CAN-06-0054. Cancer Res. 2006. PMID: 16778178 Free PMC article. - Notch signaling in mammary gland tumorigenesis.
Callahan R, Raafat A. Callahan R, et al. J Mammary Gland Biol Neoplasia. 2001 Jan;6(1):23-36. doi: 10.1023/a:1009512414430. J Mammary Gland Biol Neoplasia. 2001. PMID: 11467450 Review.
Cited by
- Radiation-induced reprogramming of breast cancer cells.
Lagadec C, Vlashi E, Della Donna L, Dekmezian C, Pajonk F. Lagadec C, et al. Stem Cells. 2012 May;30(5):833-44. doi: 10.1002/stem.1058. Stem Cells. 2012. PMID: 22489015 Free PMC article. - Cancer stem cells as a potential therapeutic target in breast cancer.
Zhang M, Li Z, Zhang X, Chang Y. Zhang M, et al. Stem Cell Investig. 2014 Jul 16;1:14. doi: 10.3978/j.issn.2306-9759.2014.06.01. eCollection 2014. Stem Cell Investig. 2014. PMID: 27358860 Free PMC article. Review. - Expression of ALDH1 in breast invasive ductal carcinoma: an independent predictor of early tumor relapse.
Zhong Y, Lin Y, Shen S, Zhou Y, Mao F, Guan J, Sun Q. Zhong Y, et al. Cancer Cell Int. 2013 Jun 15;13(1):60. doi: 10.1186/1475-2867-13-60. Cancer Cell Int. 2013. PMID: 23767668 Free PMC article. - Understanding and targeting cancer stem cells: therapeutic implications and challenges.
Chen K, Huang YH, Chen JL. Chen K, et al. Acta Pharmacol Sin. 2013 Jun;34(6):732-40. doi: 10.1038/aps.2013.27. Epub 2013 May 20. Acta Pharmacol Sin. 2013. PMID: 23685952 Free PMC article. Review. - SNAI1-mediated epithelial-mesenchymal transition confers chemoresistance and cellular plasticity by regulating genes involved in cell death and stem cell maintenance.
Lim S, Becker A, Zimmer A, Lu J, Buettner R, Kirfel J. Lim S, et al. PLoS One. 2013 Jun 17;8(6):e66558. doi: 10.1371/journal.pone.0066558. Print 2013. PLoS One. 2013. PMID: 23799116 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical