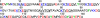Alpha-synuclein structures from fluorescence energy-transfer kinetics: implications for the role of the protein in Parkinson's disease - PubMed (original) (raw)
Alpha-synuclein structures from fluorescence energy-transfer kinetics: implications for the role of the protein in Parkinson's disease
Jennifer C Lee et al. Proc Natl Acad Sci U S A. 2004.
Abstract
Parkinson's disease is associated with the deposition and accumulation of alpha-synuclein fibrils in the brain. A30P and A53T mutations have been linked to the early-onset familial disease state. Time-resolved tryptophan fluorescence energy-transfer measurements have been used to probe the structures of pseudo-wild-type and mutant (A30P) alpha-synucleins at physiological pH (7.4), in acidic pH (4.4) solutions, and in the presence of SDS micelles, a membrane mimic. Fluorescent donor-energy acceptor (DA) distance distributions for six different tryptophan/3-nitro-tyrosine pairs reveal the presence of compact, intermediate, and extended conformations of the protein. CD spectra indicate that the protein develops substantial helical structure in the presence of SDS micelles. DA distributions show that micelles induce compaction in the N-terminal region and expansion of the acidic C terminus. In acidic solutions, there is an increased population of collapsed structures in the C-terminal region. Energy-transfer measurements demonstrate that the average DA distances for the W4-Y19 and Y19-W39 pairs are longer in one of the two disease-related mutants (A30P).
Figures
Fig. 1.
Primary amino acid sequence of human α-syn. The seven imperfect repeats are underlined. Two point mutations linked to early-onset PD (A30P and A53T) are colored gray. Red, acidic; blue, basic; green, aromatic. Residues in boldface have been either mutated (W, F, and Y) or chemically modified [Y(NO2)] for FET studies.
Fig. 2.
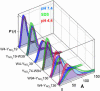
Trp-to-Y(NO2) distance distributions for freely jointed polymer chain models of α-syns under a variety of solution conditions. Green, 40 mM SDS in 20 mM NaPi buffer, pH 7.4; blue, 20 mM NaPi buffer, pH 7.4; red, 20 mM NaOAc buffer, pH 4.4.
Fig. 3.
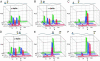
Distributions of Trp-to-Y(NO2) distances in α-syns extracted from FET kinetics. Results are shown for W4–Y(NO2)19 (A), Y(NO2)19–W39 (B), W4–Y(NO2)39 (C), Y(NO2)74–W94 (D), W94–Y(NO2)136 (E), and W4–Y(NO2)136 (F) under a variety of solution conditions. Green, 40 mM SDS in 20 mM NaPi buffer, pH 7.4; blue, 20 mM NaPi buffer, pH 7.4; red, 20 mM NaOAc buffer, pH 4.4. Center-to-center distances calculated for an ideal helix are shown as black lines in A_–_D.
Fig. 4.
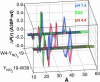
Difference distance distributions of disease-related A30P α-syns extracted from FET kinetics under a variety of solution conditions. Results are shown for W4/Y(NO2)19/A30P and Y(NO2)19/A30P/W39. Green, 40 mM SDS in 20 mM NaPi buffer, pH 7.4; blue, 20 mM NaPi buffer, pH 7.4; red, 20 mM NaOAc buffer, pH 4.4.
Similar articles
- Conformational behavior of human alpha-synuclein is modulated by familial Parkinson's disease point mutations A30P and A53T.
Li J, Uversky VN, Fink AL. Li J, et al. Neurotoxicology. 2002 Oct;23(4-5):553-67. doi: 10.1016/s0161-813x(02)00066-9. Neurotoxicology. 2002. PMID: 12428728 - Effects of Parkinson's disease-linked mutations on the structure of lipid-associated alpha-synuclein.
Bussell R Jr, Eliezer D. Bussell R Jr, et al. Biochemistry. 2004 Apr 27;43(16):4810-8. doi: 10.1021/bi036135+. Biochemistry. 2004. PMID: 15096050 - Acceleration of oligomerization, not fibrillization, is a shared property of both alpha-synuclein mutations linked to early-onset Parkinson's disease: implications for pathogenesis and therapy.
Conway KA, Lee SJ, Rochet JC, Ding TT, Williamson RE, Lansbury PT Jr. Conway KA, et al. Proc Natl Acad Sci U S A. 2000 Jan 18;97(2):571-6. doi: 10.1073/pnas.97.2.571. Proc Natl Acad Sci U S A. 2000. PMID: 10639120 Free PMC article. - Structure/function in neuroprotection and apoptosis.
Borden KL. Borden KL. Ann Neurol. 1998 Sep;44(3 Suppl 1):S65-71. doi: 10.1002/ana.410440711. Ann Neurol. 1998. PMID: 9749576 Review.
Cited by
- Protein structure evolution in liquid DESI as revealed by selective noncovalent adduct protein probing.
Moore BN, Hamdy O, Julian RR. Moore BN, et al. Int J Mass Spectrom. 2012 Dec 15;330-332:220-225. doi: 10.1016/j.ijms.2012.08.013. Epub 2012 Aug 18. Int J Mass Spectrom. 2012. PMID: 23526115 Free PMC article. - Effects of Mutations and Post-Translational Modifications on α-Synuclein In Vitro Aggregation.
Pancoe SX, Wang YJ, Shimogawa M, Perez RM, Giannakoulias S, Petersson EJ. Pancoe SX, et al. J Mol Biol. 2022 Dec 15;434(23):167859. doi: 10.1016/j.jmb.2022.167859. Epub 2022 Oct 19. J Mol Biol. 2022. PMID: 36270580 Free PMC article. Review. - Identification of fibril-like tertiary contacts in soluble monomeric α-synuclein.
Esteban-Martín S, Silvestre-Ryan J, Bertoncini CW, Salvatella X. Esteban-Martín S, et al. Biophys J. 2013 Sep 3;105(5):1192-8. doi: 10.1016/j.bpj.2013.07.044. Biophys J. 2013. PMID: 24010662 Free PMC article. - New Insights from Sum Frequency Generation Vibrational Spectroscopy into the Interactions of Islet Amyloid Polypeptides with Lipid Membranes.
Fu L, Wang Z, Batista VS, Yan EC. Fu L, et al. J Diabetes Res. 2016;2016:7293063. doi: 10.1155/2016/7293063. Epub 2015 Nov 30. J Diabetes Res. 2016. PMID: 26697504 Free PMC article. Review. - Enhanced expression of αVβ3 integrin in villus and extravillous trophoblasts of placenta accreta.
Weitzner O, Seraya-Bareket C, Biron-Shental T, Fishamn A, Yagur Y, Tzadikevitch-Geffen K, Farladansky-Gershnabel S, Kidron D, Ellis M, Ashur-Fabian O. Weitzner O, et al. Arch Gynecol Obstet. 2021 May;303(5):1175-1183. doi: 10.1007/s00404-020-05844-4. Epub 2020 Oct 28. Arch Gynecol Obstet. 2021. PMID: 33112993
References
- Parkinson, J. (1817) An Essay on the Shaking Palsy (Sherwood, Neely & Jones, London).
- Dunnett, S. B. & Bjorklund, A. (1999) Nature 399, A32–A39. - PubMed
- Spillantini, M. G., Schmidt, M. L., Lee, V. M. Y., Trojanowski, J. Q., Jakes, R. & Goedert, M. (1997) Nature 388, 839–840. - PubMed
- Clayton, D. F. & George, J. M. (1998) Trends Neurosci. 21, 249–254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous